 |
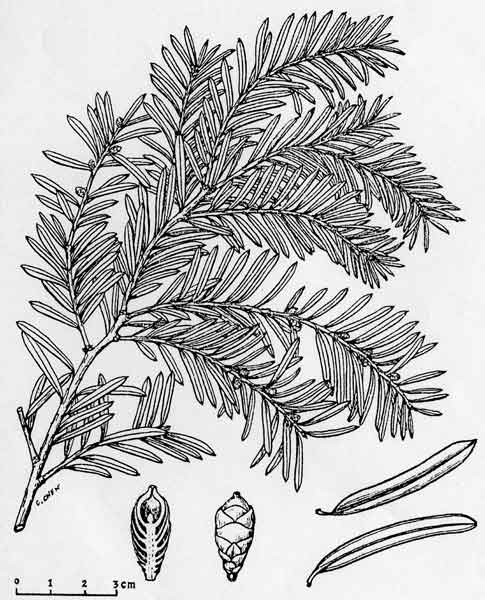 |
| Comparison of holotype of
Taxus kingstonii with illustration
reproduced from
H-l. Li., Woody flora of Taiwan, Fig. 2 (1963). |
13.
Taxus kingstonii Spjut
, J. Bot. Res. Inst. Texas 1(1): 240. 2007.
Type: China. Taiwan: Arisan Prov., Kagi, 2833 m [Mt. Alishan], 2
Feb 1918, tree 25 ft. x 2 ft., only one seen, Wilson 9738,
holotype: A! (with male cones; leaf with 10–11 stomata rows/band,
abaxial margin with 8 smooth, thick-walled, trapezoidal cells across,
anticlinal to periclinal in arrangement, followed by 5 rows of papillose
cells, midrib 12 cells wide, mostly smooth, papillose on outer 2 rows);
isotypes: BM! (leaf with 13 stomata rows and 9 marginal smooth cells,
midrib mostly smooth except upper third of leaf, papillose on outer 2
rows of cells), K! US (p.p.)!
Kingston yew.
Forest margins, or summits, 2450-2833 m in Taiwan, to 800 m on mainland
Asia; India (Khasi Hills), Myanmar, China (Tibet, Gansu, Shaanxi,
Sichuan, Yunnan, Taiwan).
Shrub
or tree to 12 m
high, bole to 65 cm diam; branchlets unequally (type) to equally
divided, yellowish green and gradually becoming yellowish with tint of
either red, orange or brown; bud scales mostly persistent, 3–4
seriate, turgid, ovate, concave, carinate near apex on upper scales, tan
to chestnut brown, lower bud-scales ca. 1.5 mm long. Leaves rigid,
spreading usually at less than right angles to branchlets, not parallel
to one another, more evenly tapered to base and apex than in T. mairei,
lanceolate, narrowly elliptical to oblong (type), or linear in other
morphs, evenly tapered to an acute, sharply pointed apex,
recurved and/or twisted downwards, 1.5–2.5 cm long, 3.0–3.5 mm
wide, 0.35–0.50 mm thick, dark green and convex above to rounded
midrib, the adaxial midrib somewhat acute in lower half, often not
evident near apex, paler green to yellowish-green and convex below, or
plane to concave below to rounded or flush midrib, often with orange
tint in the herbarium, or dull rusty brown in the herbarium, thickened
and liplike near margins, or plane and slightly revolute near margins;
upper (adaxial) epidermal cells in T-sect. wider than tall, nearly wide
rectangular or ellipitical in T-sect., usually 20–25 um tall and
25–30 um wide, thin-walled, slightly inflated; lower (abaxial)
epidermal cells similar in T-sect. but not as large, 15–25 um tall,
20–30 um wide, slightly inflated near margin in 2–5 rows, more
nearly rectangular in 7–11 cell rows near stomata bands, usually
relatively short on midrib, 1–4 (-10)× l/w, sharply 4–6 angled,
often wider and more slanted at one end (trapezoidal), not inflated (in
T-sect) as in T. mairei, papillose to ca. (7-) 8–12 (-19) rows
of cells from margins, typically without papillae on midrib (except
young leaves), or partially papillose on outer midrib; papillae
submarginal to medial in 2–3 irregular rows across each cell. Stomata
bands broader than the non-stomatal region, olive green in fresh
material; yellowish orange in dried leaves; stomata continuous in
11–15 rows, separated by 1–2 rows of accessory cells, stoma often
with a blackish halo. Male cone scales generally 4-seriate, globose, ca.
4 mm diam, yellowish-green, pollen sacs mostly 6, pale pink with reddish
mid region and patchy resinous areas. Female cone in bud subcylindric,
ca. 2 mm long, greenish, scales mostly 5-seriate, conduplicate at base,
maturing on 1st yr branchlets; seeds ovoid, dull, tan or
purplish, stained by aril, angular where tapering to apex, or not
angled, to 7 mm long, 4 mm wide.
Taxus kingstonii is recognized by the relatively turgid recurved
leaves (twist and curve downwards along their blades) that taper rather
evenly to apex, and by the rusty orange color on the abaxial leaf
surface (dried specimens). In
herbarium specimens, the leaves often crisscross, especially near apex
of branchlets, whereas leaves in other species of the Sumatrana
Group—that are reddish or greenish in color—appear more evenly
two-ranked and tightly adpressed to branchlets.
These differences seem related to the manner in which leaves
twist, which may also correlate with leaf shape as seen by the evenly
tapered leaf in the Kingston yew compared to the falcate leaf in other
species (indented more along lower margin near petiole; Fig. 20, 21, 30,
31).
Other features that help
distinguish the Kingston yew are seen on the abaxial leaf midrib (note:
27 June 2006—as seen mostly from specimens from Khasia, Tibet,
Gansu, Sichuan, and Taiwan, but not in some specimens from Yunnan).
It usually forms a rounded keel, in contrast to a truncated ridge that
may either be flush with the surface in T. celebica, or elevated
in T. mairei. Its
epidermal cells are short trapezoidal to almost rectangular, and in
T-section appear similar in size and shape to those on the adaxial
surface, or the adaxial cells sometimes are larger and nearly
isodiametric. Taxus
mairei, on the other hand, has shorter trapezoidal cells on its
abaxial midrib, but in T-section they appear larger and nearly globose—in
contrast with the shorter elliptical cells on the adaxial surface.
The abaxial midribs of the related T. sumatrana and T.
celebica have longer, nearly rectangular cells that in T-section are
similar in size on both surfaces.
The
Kingston yew also has conspicuous persistent bud-scales at the base of
young branchlets, in contrast to those of related species in the Sumatrana
Group that appear vestigial as in T. chinensis.
The Kingston yew generally
occurs at elevations between that of T. mairei (below 1200 m) and
that of T. wallichiana (above 2300 m).
Variation ascribed to T. kingstonii may include hybrids
with T. chinensis and T. mairei in Shaanzi, Gansu and
Sichuan, T. celebica in Yunnan and Burma, and T. wallichiana
in NE India. These alleged
hybrids, however, show disjunct geographical relationships as further
described in the examples that follow.
(A) Four collections, two from Shaanxi, one from
Gansu, and another from Sichuan that I had annotated T. chinensis without
microscopic examination of leaves, were later found to lack papillae on
the abaxial midrib, a feature that I employ to distinguish T.
chinensis from T. kingstonii.
Their leaves spread nearly at right angles—parallel to each
other—and have the greenish color of T. chinensis; otherwise,
they compare favorably with typical T. kingstonii. The
close morphological similarity among these specimens, along with their
more northern geographical occurrence, suggests a distinct taxon.
(B) Two collections, one from Taiwan (Liu et al.
389) and one from Yunnan (Forrest 12087), resemble T.
mairei by the isodichotomous branching, leaves spreading at right
angles, and by the thin-walled leaf epidermal cells with a yellowish
chloroplast, but are regarded T. kingstonii by the rusty orange
color on leaves and branchlets, and by the leaves ± evenly tapered to
base and apex. The abaxial
leaf epidermal cells in the specimen from Yunnan are not enlarged on the
midrib—further supporting its determination as T. kingstonii,
whereas the Taiwan specimen has slightly larger cells—indicating that
it could be assigned to T. mairei.
Yet, the closer similarity between these specimens than to other
specimens indicates a common but distinct ancestry.
(C) Collections from Yunnan (Forrest 11789, 15945,
Forrest s.n. A), Khasia (Hooker 77 P, Hooker 137 P,
Hooker & Thomson s.n. yr 1855 P) and from Taiwan (C-j.
Chang 1–6, without specific locality) resemble T.
celebica or T. sumatrana by the acuminate tapered leaf but
are referred to T. kingstonii by the leaves spreading obliquely
(Fig. 18), tapering evenly to base, having a uniformly rusty orange
color on the abaxial surface, and having short trapezoidal epidermal
cells on the abaxial midrib that in T-section are not larger than those
on the adaxial surface. On
one hand, these specimens have much in common to indicate they belong to
a distinct taxon while on the other hand specimens from Khasia (Mann
s.n. P, Fig. 19) appear intermediate to T. wallichiana by the
thicker glossy leaves, or belong to T. sumatrana, distinguished
by the thinner puckered leaves (Mann s.n., “10 ft
high”—annotated Cephalotaxus mannii, GH, Taxus baccata,
A). The Yunnan specimens
have relatively thin leaves, a character trait that may have been
acquired by hybridization with T. celebica (or T. sumatrana).
Thus, the Yunnan and Khasia plants of the Kingston yew could also
be polyphyletic.
The shape of the leaf
epidermal cells on the abaxial surface also helps separate T.
kingstonii from the T. chinensis species complex (T.
aff. chinensis in Appendix; T. phytonii Spjut ined.; T.
phytonii var. obscura Spjut ined, in adnot. A, GH, K, P, U),
particularly T. obscura ined. as defined in the preceding key.
Specimens of this species complex from Luzon and Taiwan are
remarkably similar to T. kingstonii in their leaf characteristics
of color and arrangement, but differ in more papillose cells on midrib
and marginal zones—occurring to within (8-) 4 (-2) cells from the
margins. Occasional
specimens from Luzon (Sulit 2350, Alvarey 18369
from Benquet Mt.), Fujian (Chung 3866) and Thailand (Lobb 461)
with fewer papillose cells—lacking across 8 marginal cells and often
on the lower half of the midrib—may be hybrids between T. obscura
(ined.) and T. kingstonii. They were identified T. kingstonii
in my 1996 annotations, but considered T. obscura (ined.) in this
paper by the fusiform shape of the epidermal cells on the abaxial
surface of leaves.
On the other hand a more
conservative treatment might include the Philippine yews (T. obscura
ined.) under T. kingstonii based on leaf arrangement and color, a
character that I had applied to a limited extent that resulted in other
Philippine specimens being included under T. kingstonii in my
1996 annotations (e.g., Leano 25128).
This, however, seems contrary to leaf anatomical data in this
study. The relatively
narrow border of bare marginal cells (4 cells wide), the papillose
midrib, and the 11–14 stomata rows/band occur consistently in many
specimens that would seem to merit separate taxonomic status.
It is also interesting that Ludlow & Sherriff 3719
from NE India has similarly discolored leaf surfaces with elliptical
shaped epidermal cells in T-section, 12 stomata rows/band, and a
papillose undersurface that extends entirely across the reddish marginal
zone, and that Tsai 59874 from Yunnan, and that Neth. Ind. For. Serv.
bb:20887 from Sulawesi, are also similar in this regard. Their leaf
characteristics compare more with The Philippines yews than with
those in the Himalayas. Further
study is needed to determine to what extent epidermal papillae are
influenced by genetic and environmental factors.
Taxus kingstonii is
named in honor of David G. I. Kingston, a chemist who has done extensive
work on elucidating and summarizing the taxane chemistry of the genus
(e.g., Kingston 1996; Kingston et al. 1990.
Taxol, from which the drug paclitaxel is marketed and used to
treat ovarian and other cancers, was originally isolated from dried bark
of T. brevifolia; however, fresh leaves of many species of Taxus
have proved suitable for obtaining taxanes.
Taxol was first characterized by Monroe Wall’s group (Research
Triangle Institute, Wani et al. 1971).
They also discovered other significant antitumor agents such as
camptothecin and holacanthone (Wall et al. 1972).
Representative Specimens—India:
Khasia, Hooker L77 (PH), 1337 (K) Simmons 484 (P),
Nungluai, 5000 ft, Mann (K, P). Myanmar: Bernardmyo, Ruby
Mines, 5600 ft (K). China—Tibet: Oriental, Haut Mekong,
Tsekou to Nekou, Soulie1411 (P: 2 sheets). Gansu: F. N.
Meyer 1790 ex USDA (P). Shaanxi (Shensi) Davis 1872
(P). Tsin-lin au Lao-lin,
3000 m. Sichuan:
Tachienlu Cheng 1001 (BM), Cheng 1475 (P). Yunnan:
Shweli-Salween Divide, 10,000 ft, 25º20 N, shrub 10–20 ft, open shady
thickets, Forrest 11789 (BM, K), Salween, Forrest 12087
(K, S: C-2093), Forrest s.n. (A); Ma-Chang-Kai, valley, 25º30 N,
6000 ft., shrub, 20–30 ft., in thickets, Dec 1918, Forrest 9462
(A, K), Forrest 15945 (BM, K); Salween E of Tengyueh, to summit
of Shwell, Shwelli River, Rock 7587 (US).
Taiwan: Paseian San [Pahsienshan], Hsi 165 (PH); Fig.
3 in Li, Woody Fl. Taiwan, Liu et al. 437, (M, T, US); Mt.
Ammachan, Liu 0389 (A, K), Arizan, Nitak (PH); Mt. Ammashan,
Taichung Hsien, C.C. Tseng s.n. (BH); Tongshi, C-j. Chang,
Dongshi #2, Tongshi #6, without locality data, 4 Mar 1993 (wba).
|