Taxonomic Methods and StandardsMethods Twenty-four species and 55 varieties of Taxus are identified in taxonomic keys and in detailed descriptions by morphological characters. The species concept is based on pattern recognition from study of more than 1,000 dried herbarium specimens and another ~ 300 fresh specimens from plants collected throughout the range of the genus (shipped via express next day delivery), including many cultivars at the Secrest Arboretum in Ohio, The Royal Botanic Gardens at Kew, and The Botanical Garden in Paris; data for 845 specimens are summarized in Spjut (2007a). One additional new variety of T. brevifolia was discovered in 2011 while conducting field work for Veratrum californicum in the Klamath Region of the Pacific Northwest (Spjut 2014, abstract submitted). The characters include branching (dichotomous vs. isodichotomous), color of leaf epidermal cells and stoma bands, persistence of scales at base of branchlets, bud-scale texture and size, change in color of branchlets from 1st to 2nd yr, leaf arrangement, leaf shape, leaf thickness, leaf curvature lengthwise and across both surfaces, changes in leaf curvature near margins and along midrib, shape of cones in bud and at maturity, whether seed develops on current season growth or older branchlets, shape and color of seed, shape of epidermal cells as seen in cross-section, color of leaf epidermal and mesophyll layers in leaf sections, number of marginal cells without papillae, number of stomata rows in a stomata band, shape of parenchyma cells in the leaf spongy mesophyll, and others (character list in DELTA format, research proposal on Taxus, USDA Memorandum, Spjut 1995). Several key taxonomic characters require examination of leaf sections under a microscope. Two types of leaf sections are routinely prepared. Using a single-edged razor blade and a dissecting needle to hold the leaf in place under a dissecting binocular scope, 10–20× magnification: (1) transverse (cross) sections and (2) abaxial epidermal sections 5–10 mm in length were obtained from the mid region of the leaf, illustrated as follows.
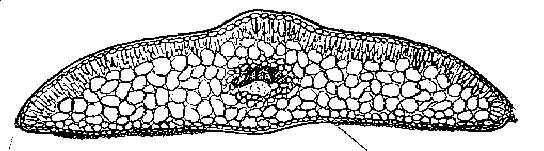
Complete transverse (T) section above and partial epidermal section below from ventral (abaxial) surface of mid region of leaf (from left margin to across midrib) of Taxus caespitosa var. latifolia, magnified approximately 100× and 250×, respectively. The upper section shows elliptical shaped epidermal cells. The lower section shows a marginal region of eight (8) smooth trapezoidal to almost rectangular cells in width with no (0) transitional zone of papillose cells; this is followed by a stomata band with 14 rows of stomata, a midrib of 15-18 cells in width that lack papillae, and part of second stomata band (1 row of stomata). The orange cells are subsidiary cells that surround the opening (“Florin's ring”). Stoma are also bordered by sunken guard cells that are not visible). Their surrounding papillose cells are referred to as accessory cells. The subsidiary cells and accessory cells are all papillose in contrast to midrib and marginal cells. The genus Taxus is defined by papillose hypostomatic bands (Dilcher 1969; Florin 1931). Top illustration drawn by Karen Parker, lower by the author. Transverse leaf sections (x-sections) of Taxus are employed to determine the shape of epidermal cells, such as elliptical vs. angular, and the number of cells that lacked papillae near the leaf margin. An abaxial epidermal section from the mid region of the leaf, generally 5–10 mm in length, is further used to determine the number of stomata rows in a band (100–250×), and the number of marginal cells that lack papillae (250–400×). Similar sketches were routinely prepared on 3.5 x 5 inch (8 x 12.5 cm) packets for most herbarium specimens studied, except that only a part of the stomata and marginal area were drawn; examples are shown in keys and under representative specimens cited for each species. A dried leaf from a herbarium specimens was usually soaked in water for at least six hours before sectioning. The mesophyll parenchyma is not always preserved in dried specimens, but this is only critical for the NW Himalayan Taxus contorta as the taxonomic features for all other species are primarily epidermal. The number of stomata rows in a band and the number of marginal cells adjacent to stomata bands were recorded on each packet. Occasionally, leaf sections were photographed using color slide film from which prints were then obtained (especially in the Wallichiana Group). The slides, photographs and packets were retained with the voucher specimens as also shown for species of the Wallichiana Group. Use of photographs is highly recommended to minimize handling of the specimens as needles detach easily. Artificial stains are not necessary because leaf epidermal sections generally exhibit a characteristic color pattern for each species. Leaves from fresh specimens, on the other hand, although easy to section, lack color distinction except when kept in a refrigerator, then, usually within a few days, subsidiary cells (cells surrounding the stomata), or only the guard cells, may turn orange. This change in color makes it easy to count stomata rows. Otherwise, the fresh color of specimens may be preserved indefinitely if stored in a frost free refrigerator or freezer during which time the specimens will gradually become dehydrated, while still retaining their original color. The distinctive color patterns in air-dried leaf epidermal sections is obviously due to chemical byproducts. The secondary compounds, however, have yet to be identified, but apparently are phenolics that oxidize slowly after a specimen is collected, except for species in the Sumatrana Group in which a change in color occurs more rapidly upon drying—within a week after collection. Resinous like substances are generally observed in cell walls of the leaf epidermis and mesophyll layers. Taxus is known to contain cyanogenic glucosides (Khan & Parveen 1987) that break down and release benzaldehyde related compounds when plants are damaged (Seigler 1991; van Genderen et al., 1996). These compounds include taxiphyllin, dhurrine, triglochinine, and isotriglochinine (Khan & Parveen 1987). The presence or absence of various flavonoid glycosides in Podocarpaceae (Markham et al. 1985) have been found to correlate with de Laubenfels (1969, etc.) morphological taxonomy of the genera and species, and biflavones have been shown to be localized in leaf epidermal cells of conifers with the aid of aluminum chloride-induced fluorescence (Gadek et al. 1984). Taxus biflavones include sciadopitysin, ginkgetin, kayaflavone, amentoflavone 7-O-methylamentoflavone in European and Himalayan species, and bilobetin and 4-O-methylamentoflavone in samples from Poland (Krauze-Baranowska & Wiwart 2003). Index Kewensis was consulted for the original publication and other taxonomic papers concerning species names and their authors. The original publication and other literature as cited were reviewed. Stafleu and Cowan (TL-2, Taxonomic Literature, 1976-1988) were consulted for information on the location of authors’ type specimens; however, most species of Taxus lacked designated types; thus, it was necessary to designate types. Abbreviations for herbaria are according to Holmgren et al. 1990). Loans from Harvard and other herbaria had to be returned before this study was completed—in June 1996. These were returned with sketches, notations, leaf fragments and Spjut's annotation labels via the US National Arboretum (NA). Because the specimens were returned prematurely—not knowing whether the study would continue—new species names and combinations were indicated as unpublished (ineditus). However, Spjut left the USDA in March 1997. As a result he was able to devote more time to the study of Taxus in World Botanical Associates. Additional loans were arranged though the US National Herbarium (Smithsonian Institution), while travel was undertaken to study specimens at other herbaria (BM, BOLO, E, K, P, PH, S, US). Taxonomic Standards and Relationships Among Genera of Taxads The genus Taxus (Taxaceae Bercht. & J. Presl, authors fide Reveal 1998) is defined by its cone and leaf morphology (Florin 1931, 1948c, 1951), in contrast to other related genera of “taxads” (Ferguson 1978; Florin 1951, 1958, 1963)—Amentotaxus (3-4 spp., E Asia), Pseudotaxus (1-2 spp., China), Torreya (5-6 spp., E Asia, N America), Austrotaxus (1 sp., New Caledonia), and Cephalotaxus (9 spp., E Asia). Taxus, and the closely related Pseudotaxus, produce a terminal seed on a lateral (secondary) short shoot (André 1956; Dupler 1920; Miller 1988; except T. brevifolia var polychaeta, which produces up to 5 ovules per secondary shoot, Spjut unpubl.) that is only partly surrounded by a loose cupular bract, whereas in other taxad genera the seed is more fully and tightly covered by the aril (Florin 1948b; Sahni 1920). Taxus leaves are differentiated from those in related taxad genera by papillose cells that define the “stomatic apparatus” (Dilcher 1969; Florin 1931, 1948c, 1951, 1958). This apparatus includes 4–8 small subsidiary papillose cells, which encircle each stoma (“Florin ring,” Florin 1931), and adjacent (accessory) papillose cells that often intergrade into a marginal region of smooth cells. The stomata develop in longitudinal rows (periclinal) within a stomatal region that, from an evolutionary point of view, is divided into two bands by a midrib. The development of stomata in rows—further differentiated by papillose accessory cells—is the most distinguishing feature of Taxus—compared to other genera of extant taxads (Florin 1931, 1951). For example, Pseudotaxus has glaucous stomata bands (Cheng 1934; Florin 1931, 1948a, 1948b, 1948c) devoid of papillae except on subsidiary cells, and it has more stomata rows, 23–28 (Florin 1948c), compared to 4–21 in Taxus (Spjut 1992, 1993, 1998). In other taxads, such as Amentotaxus and Torreya (Amentotaxaceae), the stomata bands also appear largely glaucous with papillae found on just the subsidiary cells (periclinally arranged), in contrast to the papillose accessory cells in Taxus (Florin 1951, 1958), while their stomata bands are also much narrower and more sharply differentiated than those of Taxus. In Austrotaxus (Austrotaxaceae; Nakai 1938; Florin 1958), the leaves differ notably by their long-linear shape—comparable to some Podocarpus spp.—and by their stomata dispersed evenly across the entire under-surface without clear differentiation into rows and bands, whereas the adaxial epidermal cells appear similar to Taxus in their irregularly quadrate shape. These differences, and the presence of other traits such as sclereids and resin canals in leaves of Torreya (Bertrand 1874) and Cephalotaxus, support classification of the taxads in different families (Amentotaxaceae, Austrotaxaceae, Cephalotaxaceae, Taxaceae). Also, molecular studies employing ribosomal RNA (Chaw et al. 1993, 1995) and chloroplast DNA (Tsumura et al. 1995) indicate that Amentotaxus and Torreya are more closely related to each other than to Cephalotaxus or Taxus even though their phylogenetic relationships remain debatable (Hill 1998). Papillae should not be confused with mammillae that develop singly over most of the cell’s surface—as large rounded bumps. As seen under a dissecting scope (30×), mammillae are most conspicuous along leaf margins, less so on the upper surface (e.g., T. brevifolia; Bertrand 1874), and leaf decurrencies. Mammillae are also recognized microscopically as a large bulging lens over the abaxial midrib cells of T. mairei. Papillae, by contrast, are smaller and numerous on a cell—appearing like pimples—in 2-3 distinct or concrescent rows, generally discernible only under a microscope—at least 100×, and only on the under-surface. Papillae are always present in stomata bands, gradually diminishing in prominence outside the bands towards the margins. Additionally, the dorsal (adaxial) epidermis of Taxus leaves—that lacks cellular differentiation—is similar in all Taxus species in having short pentagonal to hexagonal cells—in sharp contrast to the ventral (abaxial) epidermis that shows a wide range of taxonomic character features that are useful for delimiting species within the genus. Classification of Taxus Species in Three GroupsThe 24 species and 55 varieties of Taxus recognized by Spjut (2000) are classified into three groups (Spjut 1998, 2000; Spjut in Hageneder 2007), two of which are native to North America, the Baccata Group, represented by the Canada yew (T. canadensis) and many cultivars related to T. baccata, T. biternata, T. caespitosa, T. cuspidata, T. recurvata, and T. umbraculifera, and the Wallichiana Group, represented by the native Florida yew (T. globosa var. floridana), Mesoamerican yew (T. globosa var. globosa), and the Pacific yew (T. brevifolia), including two varieties (var. polychaeta, var. reptaneta). The Wallichiana Group is regarded ancestral to the other groups by leaves having angular epidermal cells as seen in transverse (T) section, and by having a uniform papillose abaxial surface bordered by a narrow margin of smooth cells, most often 4 cells wide. The Baccata Group, which may have been derived from the Wallichiana Group by gradual loss of stomata and papillae, differs by smaller elliptically shaped epidermal cells (as seen in T-section), and by less developed papillae on the abaxial leaf midrib. The third group, the Sumatrana Group, seems more advanced in leaves having a broader abaxial marginal zone of differentiated epidermal cells, often appearing glossy red in dried herbarium specimens. This bare marginal zone varies in width from 8–36 cells across of which nearly half of the cells nearest the stomata band may develop papillae, but this is usually not more than 12 cells wide. Two of the species groups are further subdivided into two subgroups or alliances. The Wallichiana Group includes the Chinensis and Wallichiana subgroups; the former, which is found in both Asia and North America, is distinguished by the quadrangular to tall rectangular shape of leaf epidermal cells as seen in T-section, periclinal orientation of stomata and by purplish red branchlets. This is in contrast to elliptical or wide rectangular shaped epidermal cells, anticlinal orientation of the stomata (stomata are periclinal but the stoma itself is often oriented lengthwise towards the margin, and stomata often align in transverse rows as well as longitudinal rows), and yellowish green to yellowish orange branchlets in the Chinensis Subgroup, which occurs mostly from central China to Indonesia. The other—Baccata Group—is divided into two geographical species alliances that are distinguished by whether the abaxial leaf surface between the stomata band and margin is mostly papillose (Baccata Alliance) or smooth (Cuspidata Alliance). Li et al. (2001) have shown that the North American species of the Wallichiana Subgroup (Spjut 2000b) form a strongly supported clade in contrast to a weakly support clade represented by species allied to T. cuspidata and T. baccata, which also included T. canadensis and alleged hybrids, T. x media and T. x hunnewelliana. Evidence to support this classification can be extrapolated from molecular data in Collins et al. (2003), Krupkin (unpublished), Li et al. (2001), and Vance and Krupkin (1993) despite nomenclatural differences. Although their species determinations lack standards in regard to undetermined types for most species, T. chinensis in Li et al. (2001) is interpreted by Spjut (2007a, 2007b) as T. mairei var. speciosa, based on study of specimens and communications received from the former Phyton in Ithaca, and is also cultivated at the Royal Botanical Gardens at Kew and at Edinburgh; thus, the close relationship they found between this variety and the one other specimen in their “F” clade, identified as T. mairei, would not be all that surprising. Li et al. (2001) also noted they were unable to include T. wallichiana in their study; however, an analysis of taxad genera by Wang et al.( 2000) employing RAPD showed distinct differences in bands between T. chinensis and that of T. mairei. None of the materials received from the former Phyton in Ithaca belong to T. chinensis, and none of the herbarium collections studied indicate T. chinensis is cultivated outside of China; however, a closely related species, T. wallichiana, has been in cultivation at a garden in England (specimen at BM, annotated by Spjut, April 2005). Taxus canadensis may have been derived from the Sumatrana Group based on both groups being characterized by leaves having a wide region of marginal cells, or it may have been derived from an ancestral group that included species related to T. sumatrana and T. wallichiana (Li et al. 2001). The ancestral taxa probably were once centered in NE temperate Asia and perhaps spread from there westwards into Europe and NE America where it has changed little since the late Tertiary. In temperate Eurasia, T. canadensis is difficult to recognize due to hybridization with the Baccata Group. In E Asia, T. canadensis is perhaps no longer recognizable from a T. cuspidata Alliance that appears to have evolved from hybridization between the Baccata and Sumatrana Groups. Variation and Evolution in the Abaxial Epidermis of the Taxus LeafThe ancestral leaf of Taxus is regarded as one with stomata periclinally aligned in rows across the entire abaxial surface (Spjut 1998a), which, therefore, would be entirely papillose as seen in extant yews of the E Himalayas where yew leaves may be found with up to 21 stomata rows per band with additional stomata also occurring on the midrib (Spjut 1992, 1993, 1998, 2000a,c). It is postulated that loss of stomata occurs as yew adapts to changes in climate from relatively even distribution of rainfall and temperature to more seasonal variation in less rainfall and warmer temperatures. Relationships between leaf anatomical characters in Taxus species and moisture have been suggested (Deryugina & Nesterovich 1981). Also, the number of stomata rows per band is not entirely correlated with the width of the stomata band (Nicolsi 1982); for example, the Cuspidata Alliance (in E Asia) has slightly narrower bands with as many or more stomata than species of the Baccata Alliance (Spjut 1992, 1993, 1998, 2000, 2007a, 2007b). Dempsey & Hook (2000) also gave detailed measurements on stomata aperture and stomata number per mm2 for many cultivars in the United Kingdom; a slightly higher density of stomata is evident for the representatives that appear to belong to the Cuspidata Alliance as indicated in 4 of 5 cultivars they referred to T. cuspidata, T x hunnewelliana and T. canadensis with stomata counts of 128.93, 124.39, 121.80, and 122.77 (±11.34–15.23), in contrast to 25 specimens under T. baccata with values ranging from 82.28–97.83 (±9.72–19.76) in 18 specimens, and from 102.69–119.53 (±14.24–22.03) in 7 specimens. Papillae on Taxus leaves, which are always found in stomata bands, vary in development on marginal epidermal cells. For example, in the Baccata and Sumatrana Groups, midrib and marginal cells may lack papillae entirely, or a transitional zone of papillose cells may be present. The development of papillae on midrib cells has been controversial in taxonomic delimitation of species (Kwei and Hu 1974; Spjut 2007b). This may be due to genetic and environmental factors that are not easily sorted out. Also, in many species, the abaxial midrib is more papillose in the upper half of the leaf. Variation in the distribution of papillae along the abaxial midrib has not been thoroughly studied because it requires removal of the entire epidermal layer for microscopic study, a very time-consuming task. A marginal border of smooth cells on the abaxial leaf epidermis, varying from 0–36 cells across, may evolve in several ways. One, the leaf adaxial (dorsal) surface may gradually become revolute near margins, and then the abaxial and adaxial surfaces in the revolute portion may gradually unite with the result that the abaxial surface becomes wider at the margin, which is defined by where the leaf is most acute and where the epidermal cells are smallest, often accompanied by development of mammillae. This might account for the similar shape of the epidermal cells near the margins on both leaf surfaces in species of the Baccata Alliance, T. chinensis (Pilger) Rehder, Cuspidata alliance, and T. wallichiana Zucc. A second type allegedly occurs by loss of stomata along the margin of the stomata band and then by a gradual loss of papillae on adjacent marginal cells in which the stomata band gradually narrows over time. Representative species of this second type include T. canadensis, T. sumatrana (Miq.) de Laubenfels, and T. baccata allies in the Caucasus Mountains. It is further postulated that as yew adapts from a climate that is relatively cool and moist throughout the year to one that is seasonally warmer and drier, its leaf epidermal cells become shorter, wider, and more inflated. An example is the putative ancestral T. globosa (7–11 stomata rows/band) compared with the derived T. globosa var. floridana (5–7 stomata rows/band), and the more advanced T. brevifolia (4–7 stomata rows/band); the latter differs from the former not only by fewer stomata, but also by broader epidermal cells along the abaxial marginal region. The Mesoamerican yew occurs in montane humid forests above 2000 m where seasonal variation in temperature is relatively narrow, the Florida yew near sea level in cedar swamp or riparian vegetation where summer temperatures are hot and humid and winter is cool and dry, and the Pacific yew from near sea level to above 2000 m in mixed evergreen and montane coniferous forests in a Mediterranean type of climate (hot dry summers, cold wet winters). Leaf characteristics of var. floridana appear intermediate (synapomorphy) to the related taxa in the width of the marginal cells, number of smooth cells between the margin and stomata band, the width of the stomata bands, and the number of stomata rows, but derived (apomorphy) for reduced papillae. As further evident from other paleobotanical data discussed below, and molecular data in Li et al. (2001), this relationship is clearly evolutionary, not just a random response to environmental factors. A similar evolutionary pattern is also evident with yews in Yunnan (China), involving T. wallichiana Zuccarini var. yunnanensis (Cheng & Fu) C. T. Kuan, T. celebica (Warb.) H. L. Li and T. mairei (Lemeé and Léveillé) S. Y. Hu. The abaxial epidermal leaf cells in the former (T. wallichiana) are densely papillose and long rectangular. Their loss of papillae theoretically leads to T. celebica, a species with narrower lanceolate leaves (with long rectangular, smooth cells). It further appears that distortion of leaf epidermal cells in T. celebica may have led to T. mairei, a species recognized by the oblong to elliptical leaf shape and by the development of relatively large smooth, mammillose epidermal cells on the abaxial midrib. Taxus wallichiana occurs in montane coniferous or broad-leaved forests, usually with Abies and Picea, or Lithocarpus, from 2100–3500 m in elevation in the E Himalayas to Yunnan and Sichuan. Taxus celebica, which overlaps geographically with T. wallichiana—extending into Indonesia, often occurs at elevations below 2400 m in association with a greater number of deciduous species, whereas T. mairei, which has a similar distribution, appears associated with evergreen laurophyll forests that are seasonally dry (as delimited by Hou 1983), particularly in SE China, generally found at elevations below 1600 m. The development of leaf papillae in these examples may be partly correlated with elevation and latitude, especially since papillae probably protect the stomatal apparatus from harmful ultraviolet rays (von Frimmel 1911). Thus, the ancestral type of leaf epidermis is considered to have relatively narrow cells, rectangular to hexagonal cells in shape on the abaxial surface. In adapting to warmer environments, the cells may increase in width and appear more distorted in shape (trapezoidal, wedge-shaped, or diamond shaped). Fossil leaves of Taxus from Oligocene deposits in eastern Europe, as represented by T. engelhardtii (Kvaček 1984), compare closely to extant T. chinensis (Pilger) Rehder by the distribution of papillae, and also to T. mairei by the phyllotaxy and diamond shaped cells on the abaxial epidermis. The taxa associated with T. engelhardtii indicates it occurred in a mixed mesophytic forest type with prevailing deciduous broad-leaved components ( e.g., Acer, Ostrya, Betula) but also evergreen Lauraceae, Craigia and others (Kvaček and Walther 1998). A Lower Miocene leaf referred to Taxus engelhardtii by Kunzmann & Mai (2005) is here regarded as more closely related to T. fastigiata var. sparsifolia (Baccata Alliance, not Sumatrana Group). Leaves of Taxus in North America all have less than 12 stomata rows per band, in contrast to 11 or more rows usually seen in Asia (Spjut 1992, 1993); however, loss of stomata is also evident in one E Asian species, T. florinii Spjut, that occurs in Yunnan and Sichuan. This species is more variable than its American counter species—T. brevifolia and T. globosa—in that variation in T. florinii encompasses plants similar to both American species; T. florinii has 7–11 (-13) stomata rows in relatively narrow bands bordered by a broad region of marginal cells. This is in further contrast to the related T. wallichiana with (11-) 13–21 rows of stomata in bands that extend nearly to the leaf margin, usually to 4 cells from the margin. In some specimens of Taxus florinii, the abaxial leaf surfaces may have enlarged (inflated) epidermal cells similar to those of T. brevifolia, whereas in other specimens, the cells appear sinuous as in T. globosa. Thus, one may argue that T. brevifolia should be treated as a variety of T. globosa, but the North America taxa are more easily distinguished, possibly because the genus may have been extirpated from North America during the end of the Cretaceous at which time perhaps T. globosa survived mainly in Mexico. Other examples of stomata loss and increase in cell size are seen in European Taxus canadensis var. adpressa. Leaves of this taxon usually have 5–9 stomata rows/band and broad elliptical epidermal cells in T-section, but leaves of one specimen from Slovenia had 4 (-6) stomata rows/band, and a distorted (inflated) shape in the epidermal cells. Moreover, the irregular alignment of leaf stomata, the lack of epidermal papillae, and the wedge shape to the epidermal cells as seen from surface view are similar to a fossil described from a Pliocene deposit in Bohemia, Czech Republic (Kvaček 1984). The Yugoslavia region is also one of 33 sites in the Euro-Mediterranean region with “Paleomediterranean” woody taxa known from Oligocene, Miocene, and Pliocene deposits (Palamarev 1987). Thus, the larger epidermal cells with a distorted shape and the fewer stomata in the Slovenian yew may be a relict from the former Mediterranean montane flora that included conifer genera of Pinus, Juniperus, Tetraclinus, Abies, Cedrus, Cupressus, and Picea (Palamarev 1987), many of which occurred with this Pliocene yew (Kvaček 1984). Phytogeographical Relationships in Taxus species.
Additionally, paleobotanical data (Fredericksen 1994, 1995; Kvaček 1984; Srivastava 1994), along with stomata data of extant T. canadensis (Spjut 1998, 2000, 2007a, indicate that T. canadensis may have immigrated to North America from Europe—during the Eocene across an Atlantic bridge (Tiffney 1985). Further study is needed to determine to what extent allies of T. baccata were present in Europe; the examples of Miocene and Pliocene fossils mentioned by Kvaček (1984) all appear related to T. canadensis. Taxus may have also migrated across Beringia (McKenna 1983), between North America and Asia, during the Tertiary as suggested for other conifers (e.g., Pseudotsuga, Strauss et al. 1989), or vice versa (e.g., Fokienia, McIver & Basinger 1990; McIver 1992); however, there are no extant species of Taxus in western North America that are related to the Cuspidata Alliance. Moreover, the wide diversity of yews that comprise the Cuspidata Alliance in E Asia requires time for them to have evolved as the alleged replacement taxa for the Wallichiana Group. The extant species there vary in phyllotaxy, appearing two-ranked to nearly whorled in arrangement, and in habit from prostrate shrubs to tall trees. Transitional morphs in branching, leaf arrangement and leaf anatomy (reduced or concrescent cell wall papillae) are evident between T. umbraculifera in Manchuria and T. chinensis in Hubei and Sichuan. The most logical time for their development would be during the Paleocene and Eocene. Thus, fossil species related to T. cuspidata might be expected in western North America based on the reported discoveries of other taxad genera (Amentotaxus, Torreya; Meyer & Manchester 1997; Manchester 1999) in North American Tertiary deposits. Unfortunately, fossil leaves of Taxus are rare; leaves of Taxus schornii found in a Middle Miocene deposit of N Idaho (Clarkia area, Latah Formation) are similar in anatomical features to the modern T. brevifolia in having 4-7 stomata rows per band and adjacent papillose cells that extend entirely across the midrib and much of the marginal areas (Kvaček & Rember 2000, 2007). This discovery indicates that differences in numbers of stomata rows for North American species had been established by the Miocene, lending support to the idea that present day Taxus species were established by the end of the Tertiary Period (Spjut 1998, 2007a). Other paleobotanical evidence of Taxaceae in North America includes seed. Manchester (1994) described Taxus masonii, Torreya clarnensis, and a new genus of Taxaceae, Diploporus, from seeds in the Clarno (Eocene) formation in Oregon. Distinction of Taxus masonii from related extant species is based upon bilateral symmetry of the seeds, appearing lenticular in cross-section, although seed of extant T. chinensis and T. recurvata (English yew) show tendencies to bilateral symmetry along with development of prismatic faces (see examples in photos below). European yews and T. canadensis often have a subcylindric seed shape with a smooth surface that abruptly tapers to apex, whereas seeds in other Euro-Mediterranean yews appear conical, or truncated and lobulate—shaped like a bell pepper (e.g., cv. 'Newport Media' at Secrest Arboretum, T. baccata from Iran). Within the region from W Sichuan of China—as represented by T. chinensis—to SE Russia, as represented by T. umbraculifera var. microcarpa, seeds become smaller and more conical—increasingly narrower towards apex. This contrasts with the sharply 3-4 angled ovoid seeds for T. kingstonii Spjut in the E Himalayas (see photo below), or the apical 3-4 lobed or angled seeds of T. biternata of temperate E Asia, or various subglobose seeds in the T. sumatrana Group (see examples below for T. celebica, T. mairei var. speciosa (Florin) Spjut in SE China). The relatively smaller conically shaped seed was a taxonomic feature for distinguishing T. umbraculifera var. microcarpa and T. caespitosa (Trautvetter in Maximowicz 1859; Kolesnikov 1935; Nakai 1938).
However, seed shape can also vary from conical to obconical on an individual plant in which the obconical shape is more like a deformity in development rather one of a determinate shape.
In addition to fossilized seed and leaves, Taxaceae have also been recognized from petrified wood. Prakash et al. (1995) described the new genus, Pseudotaxylon chinensis, based on one tiny wood fragment (“6 cm” x “10 µm”) from a Miocene deposit in the Shandong Province of China; the generic name was coined for its close similarity to the genus Pseudotaxus. Disjunct occurrences of yew in SE Asia (e.g., NE India, The Philippines, Taiwan, Sumatra) have all been lumped under one species or subspecies, T. wallichiana (Pilger 1903; de Laubenfels 1988), because it is generally assumed that this distribution was attained by long distance dispersal (from birds) only in recent time, whereas the North American disjunct populations are treated as different species because their associated floras generally are traced to Neogene floras (Graham 1999). But this interpretation is not consistent with phytogeographical data on leaf anatomical traits (Spjut 1998, 2007a). The fact that many plant fossils get different names from their extant counterparts is also based on the notion that species cannot remain unchanged for millions of years (see Schopf 1981 for general discussion, and comments by Mai 1998 under Sequoia abietina). Judging from a decline in conifer diversity and the apparent development of grassland vegetation by the late Miocene (Axelrod 1976; Jacobs et al. 1999), Taxus possibly attained maximum diversity in ecological species isolation by the mid Miocene (10 my). McIver and Basinger (1989) found cones similar to western red cedar (Thuja plicata) from Eocene deposits, and that it may have been derived from a complex related to an extinct species, Thuja polaris, they described from a Middle Paleocene deposit on Ellesmere Island, whereas cedars similar to T. occidentalis are not known before the Miocene. The common redwood, Sequoia sempervirens, is hardly distinguishable a former widespread species, S. abietina, recognized from the upper Eocene to Upper Miocene (Mai 1998). The oscillating temperatures due to the advance and retreat of glaciers since the Pleistocene, which has been thought to promote speciation in tropical angiosperms (Prance 1982), might have led more to hybridization and introgression without sufficient time for Taxus to evolve distinct isolated morphs. Instead, species characteristics of Taxus have allegedly become blurred through hybridization of formerly distinct Tertiary species, which may have had unique leaf anatomical traits associated with a particular habit of the plant and phyllotaxy. The Tertiary species perhaps were more distinct because they had evolved in an environment that had remained relatively stable over a long period. The climate changes during the Pleistocene nevertheless led to many new combinations of leaf anatomical traits in Taxus as individuals with different evolutionary lineages met and crossed—such as might occur between a lingering (relict) and a new (replacement) arrival. Hybrids relating to glacial cycles have been reported in other conifers (Axelrod 1986; Bobola et al. 1996; Fady et al. 1992; Wilkinson et al., 1971). Klicka and Zink (1997) concluded from DNA evidence on North American song birds that their species had originated by early Pleistocene, and that subsequent glaciation was more of an “obstacle course” for their survival. Yew species, by comparison, are likely to evolve very slowly as a yew trunk may live 3000 years (Voliotis 1986), and new trunks can regenerate (Loudon 1844) in which a particular genotype can continue to survive almost indefinitely, perhaps until a change occurs in its environment. Yew in CultivationNearly 190 ornamental cultivars of yew have been recognized (Cope 1998).
Although yew has a long history in European folklore—from the Iron Age (350 B.C., Most introduced species of Taxus in North American horticulture are related to T. cuspidata, which may include a hybrid complex referred to as T x media (Collins et al. 2003; Spjut unpublished). From a review of herbarium specimens, including the type, T. cuspidata appears more common in cultivation than in the wild. It is generally recognized by long recurved branches with stiffly ascending linear leaves that spread nearly in two ranks. Taxus caespitosa differs by shorter (oblong), more resinous leaves, that in the typical variety are distinct for their radial arrangement. Taxus umbraculifera, which includes the Hatfield yew, Hicks yew, and var. nana, differs by having some leaves reflexed downwards while others point upwards; their phyllotaxy varies from almost decussate on erect branchlets to nearly two-ranked on horizontal branchlets. Taxus biternata is easily identified by its slender biternately divided lateral branchlets with leaves in two-ranks that are more strongly revolute in the upper third when dried. Taxus media, an alleged hybrid with many nomenclatural forms (Chadwick and Keen 1976; Cope 1998; Krüssmann 1985), seems to be the name applied to any cultivated yew that cannot be identified as T. baccata or T. cuspidata, but distinction of these species was not clear before T. media was described (Hatfield 1921). This alleged hybrid (T. media) is based on Hatfield’s (1921)
recollection of various events over a period of 18 years during which time he
reportedly grew many yew plants from seed. Parental types included the Dovaston yew, an Irish
yew variant, “typical English yew,” and several “forms” of
T. cuspidata. He further indicated that since these plants were
growing nearby each other, the variation he saw among his seedlings and older
plants was best explained by their parental types having “crossed with
each other”; however, he did not indicate which parental types
were males. Also, after seedlings appeared hardy enough, he reportedly
transplanted them to fields where not all survived the more severe winters.
This included their English yew collection, among which Hatfield recognized
the “variety repandens,” “a procumbent form” as one of the
few survivors of that collection. In the figure shown by Hatfield
of the alleged type (see 1st photo below), he also stated it was raised from “mixed seed.”
Keen (1975) and Rehder (1923), however, give additional information. Keen (1975) indicated that T. media was grown from seed of Irish yew (T. fastigiata), which was believed to have been pollinated by plants of T. cuspidata. Accordingly, Hatfield, a nurseryman at the Hunnewell Estate, Wellesley MA, kept seed from a potted specimen of T. fastigiata that was to be discarded from the greenhouse. This seed, later planted—sometime around 1903—gave rise to various cultivars that included ‘Hatfield,’ ‘Browni,’ ‘Seweli,’ ‘Wardi,’ ‘Wellesley,’ ‘Wymani’ and ‘Runyan.’ The type of T. media, and that of T. media f. hatfieldii, were reportedly from this selection (Rehder 1923), while the one for Hicks yew was taken from the Hicks Nursery in Long Island (Rehder 1923) grown from seed of “cuspidata ‘nana’” that was suspected to have been pollinated by a “glauca” or “nigra” form of T. baccata. The type for T. media seems to have been selected from a hardy cultivated form of “English yew” (T. recurvata), although naturally occurring plants appear similar based on specimens from Ukraine and the Caucasus Mountains. These are related to T. recurvata by branching, persistence of bud-scales, shape of bud-scales, and leaf arrangement, but differ in their leaf anatomy in having a wider abaxial marginal zone of smooth cells. Such features are also seen in T. cuspidata or T. canadensis, but T. recurvata is distinguished by the crisscrossed leaf arrangement, in contrast to a stronger parallel arrangement in T. baccata. In E Asia, T. biternata is superficially similar to T. baccata in leaves spreading in two-ranks along one plane, but those of T. baccata and T. recurvata differ by their closer spacing in development on a branchlet, often appearing to lie on top one another in pairs when specimens are pressed. It should be noted that the phyllotaxy of a yew is always constant, while the direction in which leaves spread is often influenced by light. The Taxus media type is also similar to cv. ‘Repandens’ by the leaves turned slightly upwards near the junction of branchlets with the main branch, and by their dark green border near margins. The 'Repandens' may also be of hybrid origin as a cross between elegant yew (T. baccata var. elegantissima) and Irish yew, evidenced by the broader marginal region of smooth cells on its abaxial surface of leaves, and by leaf stomata in 11 rows per band. Leaves of Irish yew often have a broad marginal zone of smooth cells, and 11-12 stomata rows/band. Moreover, the cv. ‘Repandens’ and other “hybrid” taxa were also described at the time Rehder (Bailey 1923; Rehder 1923) described T. media. It is also possible that ‘Repandens’ may have been introduced from SW Asia. Elias and Korzhenevsky (1992) recognized a distinct shrub form of T. baccata in Ukraine and Georgia. ‘Repandens’ is a shrub type characterized by a low flat-topped habit that is generally wider than tall. In any case, the holotype for T. media does not compare closely with the plant shown in Hatfield (1921)—indicated by Rehder as the plant from which the type for T media was selected. Instead, the photo of the English yew in Hatfield (1921) is possibly T. baccata var. glauca, and and that the Hatfield's parental type may indeed be the “Irish yew variant” as also shown. Although this controversial plant has characteristics of T. cuspidata—such as the distinctive recurved branchlets and upwardly secund leaves, T. baccata var. glauca is also similar except for leaf anatomy and color. This variety is generally not known in American horticulture (Rehder 1940, 1949), but it may be found under other varietal or form names of T. baccata such as var. horizontalis Carr., var. pendula and f. semperaurea.
Other material
received as T.
media (from Phyton in Ithaca, NY) corresponds The 'Hunnewell' yew is also an alleged hybrid, T. canadensis × T. cuspidata (Rehder 1925; holotype shown here in part at right), however, T. biternata, a closely related species to T. canadensis, could also be a parental type. As with the uncertain origin of T. x media, the Hunnewell yew was thought to have arisen in 1900 on the Hunnewell Estate by chance from a seed of T. canadensis, but its hybrid origin was not recognized until 1923, and subsequently described in 1925 (Chadwick & Keen 1976; Rehder 1925). The Hunnewell morph hardly differs from cuspidata forms that have long been known in the nursery trade as “brevifolia” (Hatfield 1921), which nurseries sometimes confuse with the native T. brevifolia, whereas a cultivated shrub form of T. biternata as shown above, may be a hybrid derived in part from T. canadensis (compare also photos in den Ouden and Boom 1965 for 'Nana' and 'Hunnewelliana'); however, another morph reportedly resembles T. canadensis (Chadwick & Keen 1976; Collin et al. 2003). While there has been a lack of scientific data regarding the origin of the alleged hybrids (Price 1990), Collins et al. (2003) report evidence for paternal and maternal origins of Hunnewell yews, thus, indicating that the Hunnewell yew is indeed of hybrid origin, in contrast to the lack of hybrid evidence for one specimen included in the study by Li et al. (2001). “Various species-specific marker bands in both Taxus x media and T. x hunnewelliana is evidence for the genetic contributions from the respective parental genomes,” and that “both hybrid groups contain samples that have either one or the other parental chloroplast type, suggesting that reciprocal crosses have occurred in both hybrid groups” (Collins et al. 2003). The type for the Hicks yew (T. media var. hicksii) is similar to specimens collected in Japan, presumably from wild plants, but the Hick's yew could also have been introduced into Japan since the representative herbarium specimens cited were collected after the Hick's yew was described (Rehder 1923). It is clearly related to typical T. cuspidata (Bailey 1923) by the linear leaf, long rectangular epidermal cells, persistent thick bud-scales, and elongate leader shoots, and to T. umbraculifera or T. fastigiata, by its columnar habit and radial arrangement of leaves. Most specimens of the Hicks yew have a transitional zone of papillose cells on the abaxial surface of the leaves, in contrast to the absence of papillae in most wild specimens of T. umbraculifera. This may suggest a hybrid between T. fastigiata and T. biternata, or T. umbraculifera. In either case, the Hicks yew seems best classified under T. umbraculifera. Collins et al. (2003) found the Hick's yew to fall within their media clade in which they included samples from two plants that, however, did not pair together. The Hatfield yew, on the other hand, does not compare closely with any specimens from Japan, but its leaf arrangement is most like T. umbraculifera; it probably is a hybrid that may have included T. caespitosa as a parental type. . The Sumatrana Group is not generally in the American horticultural trade except for an introduction from seed collected by E. H. Wilson in Sichuan (China) around 650 m in elevation (Wilson 1265, A, BM, K, S, US). I have identified this and specimens cultivated from this collection at arboreta as T. mairei var. speciosa. I also recognize this same plant in leaf fragments sent to me by Phyton in Ithaca (NY). This introduction, however, has been referred to as T. chinensis (Hatfield 1921), which I consider an incorrect name as applied by Rehder (1919, 1936). The Wilson collection is representative of a polymorphic variety that is difficult to distinguish from related species, T. sumatrana, T. celebica, T. kingstonii, and the variety T. mairei var. mairei. Molecular studies of cultivars that have investigated the hybrid status of Taxus media and T x hunnewelliana are based on the phytogeographic taxonomic concept of Taxus nomenclature (e.g., Pilger 1903, Silba 1986). Li et al. (2001), in a parsimony analysis of samples from naturally occurring species of Taxus, showed a “T. x media” specimen nested with a polytomy of three T. baccata specimens, while they also reported in their parsimony analysis that it was sister to the polytomy in another of four trees. A study by Collins et al. (2003) of mostly yew cultivars, 23 of which were referred to cv. ‘Media’ among 47 different named plants of 50 Taxus samples, found strong support for the distinctiveness of T. baccata, T. canadensis, and T. cuspidata, and that T. x media and T. x hunnewelliana are of hybrid origin with all of their T. x media specimens clustered together in a dendrogram. Nevertheless, it is also interesting that Collins et al (2003) reported that none of the Taxus samples to have identical banding patterns in which they found a “surprising level of genetic diversity” in their in media group, in contrast to the low diversity level in T. canadensis samples. The relatively higher level of diversity in the media group lends support to the multiple species of Taxus within the Baccata and Cuspidata Alliances as recognized by Spjut (2000, 2007b). Further, the studies by Li et al. (2001) and Collins et al. (2003) also support Spjut's (2000b, 2000c, 2007a. 2007b) interpretation of T. canadensis, based on leaf anatomical features, as more closely allied to T. cuspidata than to T. baccata. To what extent T. umbraculifera and T. caespitosa are represented in the cv. ‘Media’ group shown by Collins et al. (2003), and their relationship to the European cultivars of the T. baccata Alliance, remains to be determined. It should be recognized that these studies considered only hybrids that might have arisen in cultivation on the presumption that natural hybrids are precluded under the phytogeographic species concept, while their investigations of T. x media were based more on the hybrid concept itself rather than on the morphological problem as tied to the type specimen. Taxoid Chemistry
The chemistry of the genus Taxus has been extensively
investigated for taxoids as a r The development of taxol to an anticancer drug has led to a taxold rush to mine for more taxane diterpenoids (taxoids), primarily since 1990. Hundreds of taxoids have been discovered in the genus, and the number continues to rise. Reviews on the chemistry of Taxus can be found in Appendino (1995), Croom (1996), Kingston et al. (1990, 1993), and Kingston (1996, 2005). Van Rozendaal et al. (1999)
presented a chemo-morphological correlation employing 18 morphological
characters and six chemical taxoid characters for 750 samples thought to be
represented by eight native species that included 66 cultivars of T. baccata,
T. cuspidata and T. x
media. But like
other chemical studies on the genus, a correlation between taxonomic
nomenclature and morphological and chemical characters as shown in most studies suffers from a lack of standards or types, and also
from the priori assumption that all species of Taxus must be geographic Another problem with taxoid chemotaxonomy is that taxoid yields vary considerably in different parts of plant, among different plants of a species, among different species, and with the analytical tools and methods employed (Croom 1995; Dempsey & Hook 2000; Griffith & Hook 1996; Hook et al. 1999; van Rozendaal et al. 2000). Variation in taxoid content may also be related to fungi that live within the plant; the fungi also reportedly produce taxoids (Stierle et al. 1993; Strobel et al. 1996), a symbiotic chemosyndrome that has also been found in other unrelated plants such as hazelnut (Corylus, Bestoso et al. 2006; Strobel 2002). SummaryThe morphological differences in Taxus appear as a product of a long evolutionary history, perhaps dating back to the Jurassic (Florin 1958). The 24 proposed species allegedly had become most distinct by the end of the Tertiary Period; subsequently, hybridization and introgression during Pleistocene has blurred their distinction. The main exception appears to be in North America where species there may have had less contact since the Tertiary, while they also may been diminished earlier by the K/T extinction event. Species of Taxus are defined by morphological characters that appear to reflect ecological differences. Leaf anatomical and chemical traits (seen by color differences) appear most useful for classifying species of Taxus into three groups, two subgroups and two alliances (Spjut 2007a, 2007b). Occasionally, anatomical traits are useful at the species level. Taxus canadensis var. minor, for example, is consistently distinct in North America for its leaves lacking papillae entirely on the abaxial surface outside the stomata bands and in having stomata in relatively fewer rows, 5–7 (-9) rows per band, than most other species. Taxus contorta Griffith in the NW Himalayas is easily recognized by its egg-shell like parenchyma cells in the leaf spongy mesophyll (idioblasts). Most species and varieties of Taxus are identified by a combination of gross morphological, color and leaf anatomical character traits such as habit, branching, persistence of bud-scales, leaf shape, and leaf arrangement, and other features. The taxonomic use of leaf anatomical traits may be further refined and guided by comparative molecular data. The extent to which DNA can be used to resolve taxonomic problems depends in part on whether it can be extracted from herbarium specimens. This was reportedly not feasible in one previous attempt (Krupkin, pers. comm. 1994), and more recently Da Cheng (pers. comm. 2007) says they cannot extract DNA from specimens submitted by the WBA that were only one year old. Many herbarium sheets are mixtures of specimens from different plants at the same location and/or from different locations, especially exsiccate; molecular data could help sort out this problem. If molecular data can only be obtained from live plants, then much data probably is already lost because yews have been discriminately and indiscriminately eliminated by Homo sapiens (Heinze 2005), and it may eventually boil down to leaf anatomy. Molecular data alone cannot decide species or varieties, and that variation in yew cannot be classified by physical geographical boundaries; morphological and/or ecological data are needed. Field work is needed to obtain herbarium material represented by both male cones—at the time of pollination—and later arillocarpia from the same yew populations. This needs to be done throughout the range of the genus. Chemical analyses might look for difference in pollen chemistry to identify perhaps chemically isolated taxa. Investigators should look for morphological variation at a particular site, and attempt to correlate molecular data with morphological traits and ecological differences such as climate, elevation, slope exposure, etc. Color photographs should be taken as an aid to document the habit and color of bark and foliage. Indeed, Gao et al. (2007) have identified 19 haplotypes in 50 populations of Taxus in China that largely correspond to known phytogeographic patterns; however, Möller et al. (2013) recognize only five species, one of which is new. They claim that Spjut's species did not correspond to their morphological data of 27 leaf characters, but two major key characters in Spjut (2007a) were not in their data set while the reference (2007a) was also not cited. One is leaf epidermal cell shape that was adopted by Hils (1993) for treatment of the yews in North America north of Mexico, and number of marginal cells on the abaxial leaf surface, which in Möller et al. (2013) is treated as a qualitative character, shininess of leaf margin.
Nomenclatural Problems
|



























 ally isolated since they cannot be distinguished morphologically.
Nevertheless, yews in the Himalayas, Europe, and Pacific northwest America have
been found to have significant chemical differences (Appendino 1995), while van Rozendaal
et al. (1999) reported that T. brevifolia is
chemo-taxonomically isolated
from other species based on its high
content of brevifoliol,
and that T. wallichiana and T. floridana are more closely related
to each other than to other species (http://www.ftns.wau.nl/oc/research/phytochemistry/Taxus/taxus.htm)..
ally isolated since they cannot be distinguished morphologically.
Nevertheless, yews in the Himalayas, Europe, and Pacific northwest America have
been found to have significant chemical differences (Appendino 1995), while van Rozendaal
et al. (1999) reported that T. brevifolia is
chemo-taxonomically isolated
from other species based on its high
content of brevifoliol,
and that T. wallichiana and T. floridana are more closely related
to each other than to other species (http://www.ftns.wau.nl/oc/research/phytochemistry/Taxus/taxus.htm)..