|
Taxus baccata Linnaeus, Lectotype (BM). Photo by Richard Spjut |
Taxus cuspidata Siebold & Zucc. |
Overview of the Genus Taxus (Taxaceae):
The Species, Their Classification, and Female Reproductive Morphology
Richard W. Spjut
First Prepared July 1999
as a brief commentary
Revised Dec. 2010 as a publication
©The
World Botanical Associates, Inc.
|
Taxus baccata Linnaeus, Lectotype (BM). Photo by Richard Spjut |
Taxus cuspidata Siebold & Zucc. |
|
Abstract. An overview is presented on the taxonomy, nomenclature, and the female reproductive morphology of the genus Taxus. The genus has a long history of its species being subjectively decided by geographical distribution patterns for which 7–11 species have been recognized to occur in temperate North America and Eurasia, extending southwards to the higher elevations of tropical southeast Asia and Central America. The discovery of the common geographically defined species are reviewed chronologically. This is in contrast to 24 species and 55 varieties defined by morphological differences, presented on this website and supported by peer reviewed publications. Recent DNA studies on Taxus are briefly reviewed in relation to the species concepts. It is further noted that species of Taxus have not been clearly understood because botanists have not followed the International Code of Botanical Nomenclature in applying the correct names. Examples of misapplied names are presented. The interpretation of the female reproductive morphology is discussed in regard to the prevailing view that a Taxus seed develops terminally on a short shoot that arises in the axil of a leaf. From further review of the literature, it is suggested that the ovuliferous shoot complex (cone) of Taxus is similar to the conifer type of cone—in that Taxus seeds develop on lateral secondary shoots arising from the axils of scales (bracts) of a primary shoot. Images and illustrations are shown of primary ovuliferous shoot complexes that have two to five ovules. The taxonomic classification of Taxus has been controversial because botanists view that Taxus seeds do not develop in cones, while they continue to include the genus and its family (Taxaceae) among the conifers in the order Coniferales. Introduction Taxus, commonly known as yew, is a genus of gymnosperms—a classification term for plants with seeds that do not develop in an ovary (“naked seeds”). Other examples of gymnosperms include Ephedra, cycads, ginkgo, and conifers, in contrast to angiosperms (flowering plants) whose seeds develop in an ovary. Among the gymnosperms, Taxus has leaves similar to that of conifers such as pines (e.g., Pinus), redwoods (e.g., Sequoia), junipers (Juniperus), cypresses (e.g., Cupressus), cedars (e.g., Thuja), and others (de Laubenfels 1953), while it notably differs from them in that its seeds do not develop in the conifer type of cone; rather, one seed is usually produced within a red fleshy cup at the end of a short scaly shoot. Whether the genus Taxus should be classified among the conifers in the order Coniferales (Quinn et al. 2002), or placed in a separate order (Taxales, Bobrov et al. 1999; Semikhov et al. 2001), which may be classified at a higher level—in a separate class (Taxopsida, Florin 1958)—because of its different cone (or lack of cone, Florin 1948, 1951; Cope 1998)—has been controversial (Pant 2000). A brief overview of the taxonomy of the species classification regarding the geographical vs. morphological species concept, the nomenclatural problems associated with these concepts, and the female reproductive morphology, will be presented. |
|
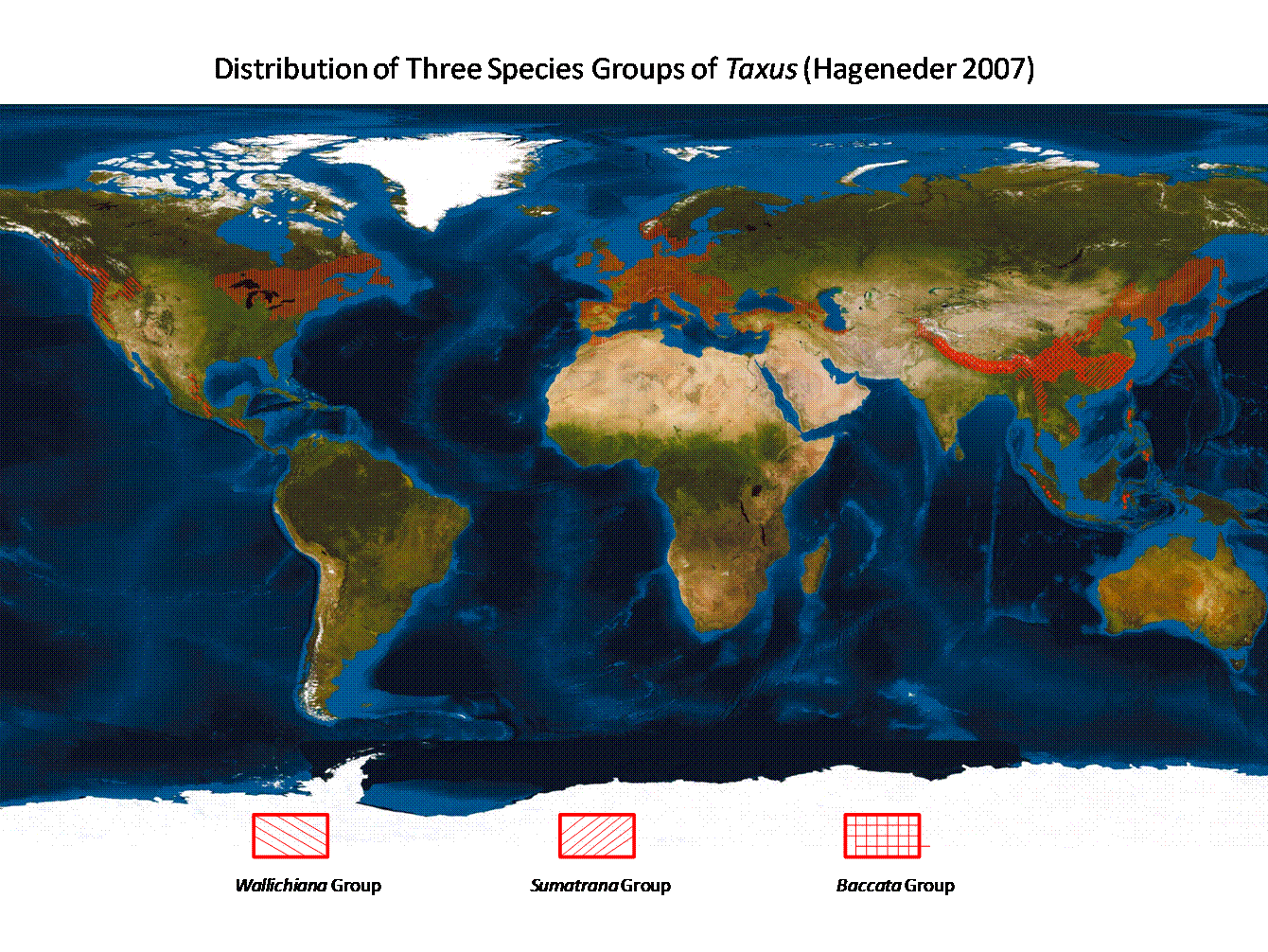
The genus Taxus includes 24 species and 55 varieties (Spjut 2000, 2007b) distributed across the northern temperate and subtropical regions as far south as El Salvador in Central America and Sumatera in Southeast Asia (Spjut 1998b, 2000c, 2007a; Fig. 1). The species are classified into three groups by differences in leaf epidermal (outer skin-like layer) and stomatal (donut-like cell) features. These are: (1) the Wallichiana Group with 11 species, occurring from central Himalayas to Indonesia (Sumatera, Sulawesi) and The Philippines, in North America in the Pacific Northwest and from Mexico to Central America with an isolated occurrence in Florida, (2) the Baccata Group with nine species in temperate Eurasia, northern Africa and eastern North America, and (3) the Sumatrana Group with four species overlapping in distribution with the Wallichiana Subgroup in Asia, but absent from North America (Spjut 1998b, 2000c, 2007b). The Wallichiana group is further divided into subgroups Wallichiana and Chinensis and the Baccata Group is divided into the Baccata and Cuspidata species alliances. An outline to the classification of the Taxus species can be found on separate web pages in keys to species groups and subgroups and by nomenclature (worldbotanical.com), in scientific papers presented at scientific meetings (Spjut 1998b, 2000c, 2010), in papers published in the Journal of Botanical Research Institute of Texas (Spjut 2007a, 2007b), and in a book on the Yew: A History by Fred Hageneder (2007). Hageneder (2007) outlines the distinctive morphological character features for each taxonomic level (taxon). Additionally, this website provides images of the herbarium specimens for the species and varieties studied, an introduction to the significance of leaf anatomical characters, the problems with the concept of Taxus x media, and to the geography of the genus. Although 24 species and 55 varieties of Taxus can be distinguished by morphological features (Spjut 2000c, 2007b, 2010), the species remain controversial in sharp contrast to conservative views that recognize only 7 to 11 species by geographical boundaries. The geographical species concept of Taxus, however, has received little scientific support except for species found in the New World (T. brevifolia, T. canadensis, T. globosa including the Florida yew) and one in the northwest Himalayas (T. contorta) where their geographical separation is strongly supported by morphological and molecular differences (Spjut 1998b, 2000c, 2007; J. Li et al. 2001; Hao et al. 2008a). History of the Geographical Species vs. the Morphological Species of Taxus. Initially there appeared to be just one species of Taxus, the European yew, T. baccata, recognized by Carl Linnaeus in 1753 (excluding Torreya [Taxus]nucifera)—the official starting point of binomial nomenclature (genus name and specific epithet)—to also occur in Canada. However, Humphrey Marshall in 1785 regarded the Canada yew as distinct from the European yew, differentiating it by name, T. canadensis, and by describing it as a shrub of low growth, in contrast to what had been generally known in Europe as a tree. Yew was soon reported elsewhere. Carl Thunberg, a student of Linnaeus, discovered it in Japan during 1775–76 and considered it to be the same as the European yew (Taxus baccata) except for the cuspidate bud-scales (Thunberg 1784). Then yew was found in the Himalayas—in Nepal—by Nathaniel Wallich in 1822—and in Afghanistan, Bhutan and northeast India by William Griffith during 1835–1841, in the Pacific Northwest by David Douglas in 1825, in Florida by Hardy Croom in 1833, in Mexico by Christian Ehrenberg in 1837, in Sumatra by Taman Teysmann sometime before 1860, and in China by Augustine Henry during 1885–86 (Spjut 2007b). These later discoveries generally led to new names for yew species and varieties such as Taxus cuspidata and T. wallichiana described by Joseph Zuccarini and Philipp Franz von Siebold in1843 for those found in Japan (T. cuspidata) and in the East Himalayas (T. wallichiana), T. brevifolia described by Thomas Nuttall in 1851 for the one found in the Pacific Northwest, T. baccata var. chinensis described by Robert Pilger in 1903 for the one collected by Henry in China, T. globosa described by Dietrich von Schlechtendal in 1838 for the one collected by Ehrenberg in Mexico, T. floridana described by Alvan Chapman in1860 for the one collected by Croom in Florida, while the yew from Sumatra was described by Friedrich Miquel in 1859 as a species in another genus, Cephalotaxus sumatrana—that was later recognized by Pilger (1903) to be the same as Taxus baccata ssp. wallichiana (Spjut 2007b). Interestingly, these species were not named until many years after they were first discovered. They were also assumed to be distinct from one another by their geographical separation (allopatric species), although characteristic features such as cuspidate bud-scales for Taxus cuspidata (Thunberg 1784; Siebold & Zuccarini 1843) were sometimes mentioned. But not everyone had treated them as different species; Joseph Hooker (1890) in the Flora of British India, for example, considered the Himalayan yew (T. wallichiana) to be the same as the European yew (T. baccata). Moreover, these and other geographical occurrences of yew, some also named, were all united under a single species, Taxus baccata, by Pilger in 1903, who then divided the species into seven subspecies: ssp. baccata, brevifolia, canadensis, cuspidata, floridana, globosa, and ssp. wallichiana, two varieties—var. chinensis and var. latifolia (under subsp. cuspidata), and 29 forms (of ssp. baccata). The latter variety was new for which Pilger (1903) cited specimens from Manchuria, Sakhalin Island and Japan. Pilger (1903) adopted the subspecies classification because he did not see clear morphological differences to justify species status. In later publications he recognized only five subspecies; ssp. globosa and floridana were placed under spp. canadensis and the number of T. baccata forms were reduced from 29 to 21 (Pilger 1916, 1926). However, Rehder (1940) differentiated the five subspecies as species in a taxonomic key based on shape of winter bud-scales, orientation of leaves (V-shaped vs. plane), and tree vs. shrub habit. Although these historical species (or subspecies) and varieties of Taxus—as recognized by Pilger (1903) and others (e.g., Farjon 1998, 2001)—have been considered biologically distinct—simply by their geographical separation from one another; their geographical classification, it seems, has been biased towards geographical distribution patterns seen in other plant genera. For example, yews on the islands of Taiwan, the Philippines, Sulawesi and Sumatera, which are clearly geographically isolated by water, all have been considered to belong to the Wallich (East Himalayan) yew, T. wallichiana (Hu 1964; de Laubenfels 1978,1988, Silba 1986, Eckenwalder 2009, Earle 2010, misapplied to T. sumatrana). The assumption has been that T. wallichiana originated on the Asia mainland and its seed was subsequently transported to the islands by birds. However, many conifer species in the family Podocarpaceae found on islands in Malesia rarely extend their geographical range as far as India as exemplified by Podocarpus neriifolius D. Don, occurring in Nepal, Bhutan, northeastern India, Myanmar, Cambodia, Thailand, Laos, Vietnam, Malaysia, the Philippines, Indonesia, Papua New Guinea, and Pacific Islands (Flora of China Vol. 4, 1999). Instead, many species of conifers found on the Asia mainland are either endemic to China or extend their range to India but not to the Philippines and/or Indonesia. Thus, it might seem logical to separate the Indonesian and the Philippine yews from the Himalayan and southwest China Taxus wallichiana as done by Farjon (1998, 2001) under the name T. sumatrana in his World Checklist of Conifers; however, it must be realized that this classification has not been supported by taxonomic keys to species of Taxus, or references to such data. Subsequently, yew plants on Taiwan have been determined to be more genetically isolated from those on the mainland (Zhang et al. 2009). This may in part be due to multiple colonization events by yew from the Asia mainland, where they may have found refuge in Taiwan from climate changes during the past 5 million years as suggested for other plant genera and species (Lu et al. 2001; Y-p. Cheng et al. 2005). This would explain the occurrence of more than one species of Taxus on Taiwan despite the island's relatively young geological age, while it may be further noted that yew seeds may not have to be transported by birds over water; the island was connected to the mainland more than once since its emergence (Yu 1995). Additionally, the Taiwan biota, in turn, has contributed to that of the northern Philippines (Esselstyn and Oliveros 2010). It is only in the northwest Himalayas where one finds a single geographically well-defined species of yew in subtropical Asia, Taxus contorta (Spjut 2007a), where clearly supported by both morphological and molecular data (Spjut 2003, 2006, 2007b; Möller et al. 2007; Shah et al. 2008). In contrast to the complex geographical history of plants in Asia, that of the North American plants is better known. Plants in eastern and western North America are associated with different floras; thus, almost no one seems to question that the Atlantic T. canadensis is distinct from Pacific Northwest T. brevifolia except Pilger (1916, 1926). Nonetheless, Spjut (1992) discovered morphologically features that do indeed distinguish them (Hils 1993), although it had been long known that the Canada yew generally differs in having both male and female reproductive structures on the same plant (monoecious) in contrast to separate plants (dioecious) in all other species. Spjut (1998a, 1998b, 2000b) also suggested that the New World T. brevifolia and T. globosa (which includes the Florida yew, Spjut 2007b) were more closely related to each other than to T. canadensis—based on shape and papillosity of leaf epidermal cells. This is in contrast to the consolidation of the Florida yew with the Canada yew by Silba (1984), evidently based on the two species being less geographically separated; the Canada yew occurs as far south as North Carolina and Tennessee. Moreover, Spjut (1998a, 2000b) found the leaf characteristics of the Canada yew to be more similar to those of the temperate east Asian T. biternata than to the other species in the New World (T. brevifolia, T. globosa). Indeed, these species relationships have been strengthened by leaf phytogeographical and molecular data (J. Li et al. 2001; Collins et al. 2003; Spjut 2007a; Hao et al. 2008a), thus, reinforcing the morphological species concept of Taxus. Ferguson (1978) had earlier indicated that too much emphasis has been placed on forcing species characteristics of taxads to fit geographical data and “that the role of geography ought to be played down.” Difficulties with the Morphological Species Concept of Taxus A major difficulty with the morphological species concept of Taxus lies in the Old World species where ecological data are needed on their reproductive isolation. The species are inferred to be reproductively isolated when the same character features are repeatedly seen at multiple disjunct locations, or where more or less distributed continuously over a wide geographical range that is congruent with other biogeographical data (Spjut 1998b, 2000c, 2007a, 2007b). Where two or more species of Taxus overlap geographically (sympatric species), they may still be reproductively isolated if they occur in different ecological habitats (parapatric in Spjut 2007a, 2007b). An example is the Kingston yew (T. kingstonii) found at lower elevations in the Himalayas than the Wallich yew (T. wallichiana), and at higher elevations in mountain ranges in China than the Maire yew (T. mairei). These differences can reflect adaptations to different climate regions such as in Taiwan where the Kingston yew occurs in the Nantou region (Wet-dry Tropical Climate) in the Pahsienshan Forest (Taichung and Nantou Counties) from 2400–2600 m along ridges with mixed conifer forests in contrast to the Maire yew occurring in the Hualien region (Temperate Rainy Climate with dry winter) at lower elevations, 1000–1800 m, in riparian forests with a greater number of broad-leaved species (data from notes on communications with C-j. Chang and specimen data of Taiwan yews online at the Natural Resources Ecology GIS Database in Taiwan). Where two or more sympatric species (overlap geographically) appear less distinct in part of their range, hybridization is a likely cause and possible reason for rejecting their species status since they may not be distinct after-all. Good biological species are supposed to have reproductive barriers to retain their inherent characteristics. They are not supposed to share. Nevertheless, hybridization is tolerated (acceptable) in many plant genera. An example is in the manzanita genus (Arctostaphylos) whose species "hybridize freely" (Munz & Keck 1959). Arctostaphylos contains 66 species with nearly half of them found along a very narrow region of the California Central Coast (Parker, Vasey & Keeley 2009). Manzanita fossils have been found dating back to the Miocene (5–23 mya), while most modern species of Arctostaphylos appear to have evolved during the past 1.5 million years (Parker, Vasey & Keeley 2009). Fossils of Taxus, by contrast—that are similar to extant species—date back much further in time, at least to the early Oligocene (28–24 mya; Kvaček 1984; Spjut 2007a), and older relatives have been found in Jurassic deposits (200–145 mya) as represented by the extinct genera Paleotaxus and Marksea (Florin 1951,1958; Harris 1976b) that are referred to as “taxads” because of their apparent development of an arillocarpium as defined later. Evolution of species is evidently slower in Taxus (Spjut 2007a, 2007b) than in Arctostaphylos. Consequently, much of the morphological variation in the Euro-Mediterranean and southeast Asian Taxus has been suggested to be the result of introgression between species that were formerly more distinct during the Tertiary (Spjut 2007b). The morphological variation in Taxus kingstonii, for instance, may be partly attributed to its periodic contact during the past two million years with other related species (T. celebica, T. chinensis, T. mairei, T. sumatrana, T. wallichiana), ranging from the Himalayas to Taiwan (Spjut 2007b)—as a result of climate change related to glacial cycles as suggested for the conifer genus Cunninghamia (Lu et al. 2001). Numerous hybrids between European and east Asian temperate species of Taxus have been recognized in cultivation (Cope 1998; Collins et al. 2003), most of which are relegated to T. x media (Chadwick & Keen 1976), a name established by Rehder in 1923. It is interesting to note that many cultivars of Taxus produce abundant seed in cultivation, but rarely do they produce seedlings in the wild. Other factors that influence taxonomic decisions are previous taxonomic history and what makes the most biological sense to distinguish the species. The genus Taxus has a long history of its species (or subspecies) differentiated geographically, whereas Arctostaphylos species and varieties have always been defined morphologically. A Flora of California by Jepson (1919) initially recognized 25 species of Arctostaphylos; that number more than doubled in later floras, e.g., 57 species in The Jepson Manual (Hickman 1993). A justification for recognizing many species of Arctostaphylos along the Central Coast is the relatively few manzanita species that occur along the Sierra Nevada over a broader geographical range (Jepson 1919). A similar rationale is made by Spjut (2007a, 2007b) for the greater diversity of Taxus in southeast Asia than in North America. Like Taxus, species of Arctostaphylos are notoriously difficult to identify. Unlike manzanita taxonomy, there is a bandwagon of lumping all the occurrences of the subtropical Asian Taxus under 1 or 2 species, in contrast to many species and varieties recognized by Spjut (1998b, 2000c, 2007b) in that region. Misnomers in Taxus. The names given to species of Taxus have not been consistently applied. Although Pilger's (1903) geographical classification of the subspecies has been followed by many botanists—usually at the species level, the names applied are not always appropriately correct—according to the International Rules of Botanical Nomenclature (ICBN) as further exemplified in the paragraphs that follow. Examples include de Laubenfels (1978, 1988), Silba (1986), Eckenwalder (2009), Earle (2010) and others who have recognized one species of Taxus for the southeast Asian region—ranging from the central Himalayas to Sumatera—under the misapplied name T. sumatrana, whereas the earlier name, T. wallichiana, is the one that must be adopted. Additionally, deciding on the correct name can be complicated by other species of Taxus recognized to occur within the same region (sympatric species, e.g., Florin 1948; Cheng & Fu 1978; Spjut 2000c, 2003, 2007a, 2007b). Further differentiation of Taxus species—based on morphological grounds—has led to new species names that have since turned out to be superfluous such as Taxus yunnanensis described by Cheng and Fu in 1975 for what they had considered to be a new species occurring in eastern Himalayas to southwestern China, when T. wallichiana must be the name applied for this yew. Cheng & Fu (1975, 1978) had recognized two species of yew in the Himalayas instead of just one (T. wallichiana): (1) an eastern species that extends to southwestern China and (2) a northwestern species in which they associated with the already established T. wallichiana. This raises the question as to how does one decide which Himalayan yew goes with which name? The International Rules of Botanical Nomenclature (ICBN; e.g., Greuter et al. 2000; Vienna Code 2006), updated every six years by the International Botanical Congress, provides the rules on deciding legitimacy of botanical names, but botanists do not always adhere to the ICBN, unfortunately (e.g., Hao et al. 2008b; Shah et al. 2010). The ICBN sets forth six principles: I—independence from zoological and bacteriological nomenclature. II-—application of names are determined by types, III—nomenclature is based on priority of publication, IV—only one name can be correct for a defined taxon, V—scientific names are in Latin, and VI—rules are retroactive unless expressly limited. Two of the six ICBN basic principles apply to the preceding example, the Principle of priority (Principle III) which essentially says that the species name is to be adopted from whoever named it first (assuming that other criteria are also met under Principles III and IV), and that it has to be based upon a standard—a type specimen, or an illustration may also serve as a type if no specimen is available (rules under Principle II). Taxus wallichiana, described by Zuccarini in 1843 (in Siebold & Zuccarini 1843), is obviously earlier than Taxus yunnanensis described by Cheng and Fu in 1975, but knowing that bit of information does not tell us where in the Himalayas the specimens for the earlier name T. wallichiana originally came from.
As noted earlier, Taxus wallichiana was first collected by Nathaniel Wallich in Nepal during 1822 as determined from a review of herbarium specimens and references that represent “original material” of which there are four kinds in this particular case: (1) the collection numbers in his Numerical List (catalogue) for species names assigned to plant specimens (Wallich 1831; Anonymous 1913), particularly number 6054a, “Taxus nucifera Kaempf.?” from Nepal, (2) Wallich's report of its occurrence in Nepal in his Tentaman Florae Nepalensis (Wallich 1826, “Sheopore,” near Kathmandu), (3) Siebold and Zuccarini's (1843) account of Wallich (1826) reference to Taxus nucifera Linnaeus (1753) for which they reported that this plant (from Japan) that Linnaeus (1753) had named was really not a Taxus but a Torreya, a genus that was not recognized until 1838 by George Arnott for another Torreya species discovered in Florida (Torreya taxifolia), and that Wallich's Taxus was indeed a new species of Taxus, which Zuccarini then described and named Taxus wallichiana, and (4) the specimens Wallich assembled and distributed of Taxus that were consulted by Zuccarini from which he also prepared and published an illustration (in Siebold and Zuccarini 1843).
Wallich's Numerical List (1831) included also 6054b from “Kumaon” (a region in northern India adjacent to the western border of Nepal) with reference to the collector “RB” (Robert Blinkworth); however, there was some confusion in the numbering or labeling of duplicate specimens of 6054a and 6054b (Spjut 2003, 2007b) that were distributed to different herbaria. Of those studied by Spjut (2003, 2007b), most 6054b specimens were determined to belong to another species of Taxus, one that was also found in Afghanistan by William Griffith during 1839–1841 from where he had collected a specimen and then later described it as a new species, Taxus contorta (Griffith 1848, 1854), a species that has since been found to be widely distributed in the northwest Himalayas (Spjut 2003, 2007; Möller et al. 2007; Shah et al. 2008). This mix-up in specimens numbered 6054a and 6054b further justified Spjut's (1995, 2003, 2007b) selection of a type specimen (from uncited specimens or isosyntypes) from northeastern India (most likely in Khasia), a specimen that Wallich sent to Zuccarini in 1835, which was a good match for the illustration provided by Zuccarini (in Siebold and Zuccarini 1843); the Wallich specimen from northeastern India in the herbarium at München was annotated lectotype by Spjut in 1995 (Spjut 2003, 2007b). A detailed account of the nomenclatural problems of Himalayan yews was provided in a manuscript submitted for publication in 1998, and after a number of peer reviews, it was finally accepted in 2006, while it had also been earlier placed online (Spjut 2003, worldbotanical.com). Because most type specimens for Taxus species names had not been clearly determined (before Spjut 2007), this has undoubtedly played a factor to other superfluous names and to misinterpretations of those already published. For example, in 1997, Nan Li and Robert Mill realized that while Taxus wallichiana should have been the name applied to the eastern Himalayan yew (not T. yunnanensis), they overlooked earlier names for the western Himalaya yew such as T. contorta (Griffith 1848, 1854) and T. orientalis (Bertoloni 1862), and as a result—in thinking that they had a new species—they created a superfluous name, Taxus fuana (for a species that already had a name). Moreover, de Laubenfels (1978, 1988) earlier had apparently decided that the northwest Himalayan yew belonged to T. wallichiana since he applied the name T. sumatrana to all yews found from the eastern Himalayas to Sumatera. This confusion has led other authors to refer to the northwest Himalayan yew by its legitimate name in one paper, T. contorta (Hao et al. 2008a), and by an illegitimate name in another paper, T. fuana (Hao et al. 2008b; see also Appendix 4 and title in Shah et al. 2008).
Thus, to achieve a clear and consistent understanding as to what constitutes the species in the genus Taxus, their published names and type specimens must be clearly accounted for when circumscribing them as well as describing new species, all of which must be done according to the ICBN, which does not give priority to species named after individuals with outstanding accomplishments (e.g., Taxus fuana illegitimately named in honor of Li-kuo Fu). And those who publish molecular studies on Taxus species also need to keep this in mind. Prior to Spjut (2003, 2007b), types for most species names in Taxus had not been designated; thus, information in the literature on most species of Taxus lacked taxonomic standards. Even the type for the Euro-Mediterranean yew, Taxus baccata, had remained undetermined—until 1993—when it was finally designated (Jonsell and Jarvis in Jarvis et al.(1993), 200 years after Linnaeus (1753) had described it. This is not an uncommon problem in many plant genera as the ICBN did not adopt a type specimen requirement until 1958, keeping in mind that “The Rules of nomenclature are retroactive unless expressly limited” (Principle VI). There has, however, been recent attempts to find a legitimate basis for previous illegitimate names of Taxus, particularly T. fuana, a superfluous name as already indicated for the northwest Himalayan yew, T. contorta. Farjon (2001)—who as a curator was likely aware of Taxus specimens at Kew annotated by Spjut in 1997 and his proposed papers (Spjut 1998, 2000)—would seem to have arbitrarily decided that T. fuana is endemic to the type locality in southwestern Tibet (near the border of Nepal). However, the type specimen is morphologically not unlike other specimens from the northwestern Himalayas—Afghanistan, Pakistan, southwestern Tibet, India, western Nepal, and Bhutan (Cheng & Fu 1975, 1978; Nan Li & Fu 1997; Fu & Mill 1999; Spjut 2006, 2007b; Möller et al. 2007; Hao et al. 2008a; Shah et al. 2008)—that belong to T. contorta (Spjut 2003, 2006, 2007b; Hao et al. 2008; Shah et al. 2008, Appendix 4). Moreover, other collections made from the type locality—that were employed in DNA studies—have not reported any unusual genetic traits (Hao et al. 2008a, 2008b). The USDA ARS Germplasm Resources Information Network (GRIN, accessed Jun & Oct 2010 ) has also attempted to justify T. fuana by suggesting that T. contorta is restricted in geographical occurrence to Afghanistan (type locality), or by citing it as a synonym of Taxus wallichiana var. wallichiana. This latter interpretation is then contradicted by their own geographical data—indicating that T. wallichiana does not occur in Afghanistan. Thus, their accepted species of Taxus imply new interpretations without supportive references, including also T. sumatrana interpreted as found in Indonesia and the Philippines (Farjon 1998, 2001; GRIN, accessed Oct 2010) as well as T. fuana restricted to southwestern Tibet (Farjon 1998, 2001). In regard to the nomenclatural reviews by GRIN, their scientists do not consult type or other specimens in herbaria for determination of the correct name. Their consensus on the accepted species has been strictly literature based. Previous illegitimate names of Taxus have nonetheless found legitimate application as a result of a comprehensive review of the genus by Spjut (2003, worldbotanical.com; 2007a, 2007b). Taxus chinensis, for example was illegitimately established by Rehder in 1919 as his choice over an older name he serendipitously discovered under another genus, Tsuga mairei, described by Lemée and Leviellé as a new species of hemlock (Leveillé 1914). Rehder (1919) recognized the type specimen to belong to Pilger's Taxus baccata var. chinensis, but also decided that this variety deserved species status. However, the ICBN requires that the earlier species epithet, mairei, be adopted, not the varietal epithet chinensis even though it is an earlier name. This is because the priority rule applies only to names within the same rank (ICBN Art. 11.2); species and varieties are different ranks. The type specimens for both names were considered by Spjut (2003, 2007) to belong to different species based on morphological differences, thus, he found a legitimate basis for them.
Another example is Hao et al. (2008a) who recognized Taxus yunnanensis as distinct from T. wallichiana with reference to Spjut (2007b), which it helps to cite this reference because the application of these names have been confused by others in the past as already explained above. Authors who draw conclusions on species DNA and chemistry in the genus Taxus should include geographical and morphological information about the plants sampled and also provide images of voucher specimens so that others can more readily determine how their information compares to the type specimens. Unpublished Species and Varieties of Taxus Not all the proposed new species and varieties reported on worldbotanical.com have been published. Keys to all the proposed species and varieties were presented at joint meetings of the Botanical Society of America and American Systematic Plant Taxonomists, held in Portland, OR in 2000, distributed in late 2007 to most institutions who provided loans of herbarium specimens during the mid 1990's, and sent as three pdf files in March 2010 via email for presentation at the Third International Conference on Yew at Ponferrada, León, Spain. In June 1996, Spjut had found it necessary to annotate specimens of Taxus by his unpublished names because of uncertainty that he had had at that time as to whether he would be able to continue his research on Taxus. The United States Department of Agriculture (USDA) Agricultural Research Service (ARS) had decided in Nov 1995 that the specimens Spjut had obtained on loan had to be returned. Generally, loaned specimens are returned with annotation labels. This included loans obtained for another genus, Amaranthus, also under study by Spjut. Specimens for one loan of Amaranthus—selected because they had been previously studied by Jonathan Sauer, a long time and well-known specialist on the genus—were not annotated. After their return, the curator sent a letter complaining that the specimens were returned without annotation labels; however, Spjut's purpose in this case was to learn Sauer's concept of the Amaranthus species, not to revise his taxonomy. A large number of Taxus specimens on loan from the HUH and other herbaria, obtained via the U.S. National Arboretum (NA), were annotated during June 1996 before return. Soon afterwards, Spjut was then persuaded by the USDA to take an early retirement, while they also denied him an award from the NIH to collect plant samples throughout the United States for cancer research. Upon retiring in March 1997, Spjut then continued his research on Taxus that included visits in Oct 1997 to the Museum of Natural History in Paris, the Museum of Natural History in London, and the Royal Botanic Gardens at Kew, where at all three institutions he annotated and photographed more specimens of Taxus. At that time Spjut was also a Research Associate of the Smithsonian Institution where he photographed and studied their (US) specimens and received assistance from them to obtaining additional loans of Taxus from the Swedish Museum of Natural History. Furthermore, it may be noted that Spjut had begun a taxonomic study of the genus Taxus as part of his duties in collecting plant samples for the National Cancer Institute (NCI) during the mid 1970's as an employee of the USDA ARS. The initial investigation focused on T. brevifolia to determine where the ARS could procure tons of stem-bark needed for clinical trials of the anticancer compound taxol (Spjut, USDA Memorandum 1977). An extract from the bark of this species was discovered to have antitumor activity in 1964. This led to isolation of taxol and evaluation of its pharmacological activity (Perdue & Hartwell 1969; Wani et al. 1971). In 1992, taxol was approved by the Federal Drug Administration as an anticancer drug. The continued need for taxol has led to search for alternative sources of taxol (International Yew Resources Conference 1993; Croom 1995). Goodman and Walsh (2001) provide a detailed account of the “nature and politics in the pursuit” of taxol. Molecular and Chemotaxonomic Studies on Taxus Molecular DNA and chemotaxonomic studies of species in the genus Taxus have largely assumed that the species have a solid taxonomic foundation, generally according to the geographical parameters laid out by Pilger (1903). As already noted earlier, most species names of Taxus lacked standards in regard to type specimens—until they were designated in 2007 by Spjut (2007b). It may be argued, therefore, that it could be anyone's guess as to what chemical or molecular data goes with which species name. Examples are publications on Rearranged taxane from the Himalayan Yew Taxus wallichiana, by B. Das, S. Padma Rao, K. V. Srinivas, J. S. Yadav and R. A. Das in Phytochemistry 42 (3), 787-788, 1996 and Taxoid from Needles of Himalayan Taxus baccata in Phytochemistry 38, 671-674, 1995 by the same authors. Did the authors report on two different species of Taxus in the Himalayas or just one? The nomenclature problem for Himalayan yews was first addressed in detail by Spjut (2003) and later mentioned by Shah (2010). Although voucher specimens are usually cited in chemical and molecular studies, the relationship of a voucher specimen to the type specimen cannot be easily determined without morphological data, or reference to such data. This is especially a problem for DNA studies because extraction of DNA from Taxus generally requires fresh material (Spjut 2007b), whereas most type specimens of Taxus were collected more than 100 years ago. On one hand, advocating a geographical species concept of Taxus bypasses this problem, while on the other hand, one would expect samples collected of a genus in different geographical areas to show geographical differences on a molecular level. This is also to say that conclusions drawn from molecular taxonomic data on Taxus species need to be corroborated by morphological data, a chicken and egg problem generally overlooked. Nonetheless, molecular studies of Taxus have contributed to elucidating the evolutionary genetic (phylogenetic) relationships among the various entities that have been sampled. For example, Collins et al. (2003) reported they found considerable genetic diversity in cultivated yews represented by species of the Baccata and Cuspidata Alliances (Spjut 2000c, 2007b) for which they cited T. baccata, T. canadensis, T. cuspidata, and numerous cultivar names under “T x media.” Their genealogical tree, or “dendrogram,” based on “unweighted pair-group method algorithm (UPGMA) analyses of RAPD bands” showed T. canadensis and T. cuspidata to be like cousins to each other, which are technically referred to as sister groups in one clade (immediate family) in contrast to the other T. baccata clade, which included a sister group to the T. x media cultivars. However, it should also be noted that they recognized three chloroplast types from which they reported the T. baccata and T. cuspidata types were present in the T. x media cultivars. Jianhua Li et al. (2001) provided a broader geographical coverage that included samples from all North American species, and also samples from a few Asian species—cultivated in arboreta. They found a strong relationship within a North American clade comprising the Pacific yew, Mesoamerican yew and Florida yew, and that the Canada yew was sister to other Eurasian yews, all belonging to a larger less strongly supported clade. Hao et al. (Biol. Pharm. Bull. 31(2) 260-265 (2008) included more species native to China. Their data were backed by images of some voucher specimens to substantiate their findings even though the black and white photos lacked high contrast. While their identifications do not agree entirely with Spjut (2007b), their T. baccata appears to be T. baccata var. washingtonii, their T. cuspidata is T. umbraculifera var. umbraculifera (Cuspidata Alliance of Spjut 2007b), their T. sumatrana (origin unknown) was from a cultivated plant in an arboretum at Atlanta, GA, which is possibly T. mairei var. speciosa (Sumatrana Group of Spjut 2007b); their T x media appears to be a common hybrid of species in the Cuspidata Alliance and their T. mairei is probably T. kingstonii. In regard to the latter determination, leaves in T. mairei are less tapered near petiole and abruptly tapered to apex, and while this could also be T. chinensis, its position on their “combined tree” agrees more with T. kingstonii (Sumatrana Group of Spjut 2007b). Specimens not represented by images included geographical data on where samples were collected. From these data it would appear that their species determinations agree with Spjut's (2007b) taxonomy except for nomenclature in regard to T. yunnanensis; they argue that it should be kept as a separate species in contrast to Spjut's treatment of it as a variety (T. wallichiana var. yunnanensis). Their results (Hao et al. 2008) were presented in a “combined tree” that showed a stepwise series of clades—in a lineage—leading to a clade that branches further—ultimately into “subclades,” one containing the New World species and another the Old World species of the Wallichiana Subgroup of Spjut (2007b); a sister clade to the latter includes species of the Sumatrana Group (T. sumatrana, T. kingstonii [as T. mairei], and T. mairei; Spjut 2007b). The temperate Eurasian species and northwest Himalayan T. contorta grouped together in a larger clade corresponding to the Baccata Group of Spjut (2007b) for which the Canada yew is basal. The North American Wallichiana Subgroup of species agrees with J. Li et al. (2001), except in the topology. Their T. chinensis, represented by two samples in a clade from “ShenNongJia” and “YunXi” in Hubei, did not cluster with T. wallichiana and T. yunnanensis, which were represented by samples from southeastern Tibet; instead, they were in a separate clade. Taxus chinensis was treated as variety of T. wallichiana by Fu & Nan Li (1999) in contrast to Spjut who recognized a Chinensis Subgroup within the Wallichiana Group. Spjut (2007a) also suggested T. chinensis may have evolved as a result of introgression involving species allied to T. cuspidata and T. contorta var. mucronata; thus, their (Hao et al. 2008a) results are perhaps closer to a true phylogeny. A major weakness in phylogenetic studies of Taxus is the lack of a broader geographical representation for each of the species upon which conclusions are drawn. Inferences are being made on species assumed to be widely distributed; yet, sampling of the yew DNA is geographically limited. If a broad geographical species concept is to be applied then the species should include samples throughout its range, not just from one or two locations. For instance, the samples cited by Hao et al. (2008) for both varieties of Taxus wallichiana were from southeastern Tibet; thus, one might expect that they would group in a clade, especially since the comparative data of Taxus samples were from other regions. To have more realistic meaning at the species level, samples of these varieties should also have been collected from Yunnan and Sichuan, while it may be noted that the Tibet locations are closer to the type localities for both varieties. Whether one agrees or disagrees with Spjut's (2007b) taxonomy, an effort should be made to include samples of morphological variation that was recognized in this publication to better assess the genetic variation, phylogenetic and phylogeographical relationships in the genus Taxus. Reproductive Shoots of Taxus Besides taxonomic and nomenclatural confusion in Taxus, the terminology for the reproductive (strobilar) shoots of Taxus varies according to one's interpretation of how they compare with other gymnosperms (Miller 1988; Cope 1998). In overwhelmingly much of the literature, the female (ovule-bearing) shoot of Taxus is reported to bear a single terminal ovule, which at maturity the seed becomes loosely enveloped by a fleshy cup known as an aril (Fig. 2); together they constitute the arillocarpium (Spjut 1994). However, more than one ovule may be found on a reproductive shoot, in which the ovules do not always appear terminal as further explained below. |
|
Fig. 2–4.
Fig. 2. Two top images. Secondary ovulate shoots (cones) of Taxus
brevifolia var. reptaneta. Left: Spjut & Broster 12308,
Flathead National Forest, Montana. Right: Spjut 12301, Eastern slopes of
the Cascade Range, near Timothy Lake Oregon. Both specimens photographed Aug
1992. Fig. 3 (Lower left): From Dupler (1920. "Figs." 1–5),
including caption; arrows and labels added.
The structure of the Taxus ovuliferous shoot is not quite as simple as it may appear. Initially a primary shoot develops in the axil of a leaf, beginning with several transversely arranged scales followed by a series of spirally arranged scales from which a secondary shoot arises in the axil of one of the uppermost scales (Strasburger 1889, Fig. 4; Roberston 1907; Pilger 1909, 1916, 1926; Dupler 1920, Fig. 3; André 1956, Fig. 5). Dupler (1920) characterized the “ovuliferous organ” of Taxus to “consists of the primary shoot and the secondary shoot with the ovule” (emphasis added). The compound nature of this structure is not readily apparent because of its small size and initial development within bud, which upon sectioning, the separate development of the primary and secondary shoots can be seen (Fig. 3–5).
|
|
Ovule emerging from axil of scale
|
|
Fig. 6–7. Fig. 6 (Left): From Pilger (1916), showing various shoots of “Taxus baccata.” Note shortly branched bi-ovulate shoot in lower left illustration (d) and in right illus. labeled (b). Fig. 7 (Right): From Pilger (1926). Simple and multiple (“lateral”) secondary ovular shoots of “Taxus baccata.” Numbers refer to pairs of bracts on the secondary shoot to illustrate their decussate arrangement. Below the numbered bracts are scales of primary shoot. Arrow added to denote ovule(s) emerging from the axil of scale (x1) fide Pilger. Note the two transverse scales at base of primary shoot designated in Pilger's Fig A. Although Fig. 7 is reproduced here from Pilger (1926), this same figure was indicated by him to have been published earlier (Pilger 1909, n.v.). |
|
The secondary shoot develops three pairs of bracts overlapping in a cyclic arrangement (decussate, Fig. 7), while the overall number of scales and bracts on the primary–secondary shoot complex ranges from 12–20 (Cope 1998), or >20 in T. brevifolia var. polychaeta. The sporophyll nature of bracts (evolutionary origin from a leaf) is evident by the presence of chlorophyll and stomata (Dupler 1920). These are not homologous with ovuliferous scales of conifer cones that are regarded to have evolved from a shortly branched shoot complex (Chamberlain 1935; Florin 1951; Wilson 2005). The sterile bracts (scales) otherwise appear similar to the “bud scales” of vegetative shoots, except for their decussate arrangement, in contrast to the spiral arrangement in vegetative buds, while it may be noted that bracts (scales) on the male (pollen) shoots are also spirally arranged except for a basal pair (Dupler 1919, 1920). The secondary shoot, or entire shoot complex, has also been referred to as a dwarf shoot, an axillary shoot, seed cone, ovuliferous shoot, ovulate strobilus, or megastrobilus (Cope 1998). |
|
In most species of Taxus, the secondary shoot usually appears as a simple (unbranched) structure; however, a primary shoot may produce more than one secondary shoot (Strasburger 1889; Robertson 1907; Pilger 1909, 1916, 1926; Chamberlain 1935; André 1956), Fig. 6–10, usually where one of the two subterminal ovular shoots normally aborts development (Robertson's 1907 Fig. 4). Thomas and Spicer (1987) stated that “[t]he common occurrence of two ovules per shoot in Taxus is explained by there being two axillary short shoots in the overall fertile shoot complex;” however, as many as five ovular shoots may be found (André 1956, Fig. 8; Spjut & Baye 16710, Fig. 10, T. brevifolia var. polychaeta. Fig. 8. From André (1956). Primary ovular shoots of “Taxus baccata” with five terminal secondary shoots. brg = bud of primary shoot. A0 = primary shoot showing its axillary origin to a leaf in C.
|
|
Terminal secondary shoots may
be equally developed as shown in Pilger (1916, 1926) and André (1956) for
“Taxus baccata” (Fig. 6–8) and in occasional plants of T.
brevifolia vars. brevifolia and reptaneta, or some of
the secondary shoots may be less developed in T. brevifolia vars. brevifolia and
polychaeta (Fig. 9, 10). In
one plant of
T. brevifolia var. brevifolia,
the shorter of the two in a ovular shoot complex
also appeared lateral and partly submersed in the shoot
axis among the lower scales (Fig. 9). |
|
|
|
Fig. 9. Taxus brevifolia
var. brevifolia, Spjut 16716 (wba), from Humboldt Co., CA, (July
4, 2010) |
  |
|
Fig. 10A-B. Taxus
brevifolia var. polychaeta, Spjut & Baye 16710, from Sonoma
County, California (June 2010).
A. Left image shows two subterminal ovules on a
primary shoot. Right image shows a primary-secondary shoot
complex with five
ovules, each ovule appearing as a short lateral branch (brachyblast). Both
kinds of shoots found
on the same plant. Note the relatively short
secondary shoot compared to the long primary shoot, in contrast to the
ovulate
shoot complex seen in var. brevifolia shown in Fig 9. The image
below (Fig. 11) shows a primary shoot with
two arillocarpia from the same
location. Studies of the ovular shoot complex of T. baccata report
that multiple
secondary shoots can arise from a persistent primary shoot over a
period of several years or more. Here it also evident
that several new secondary
shoots may arise from a primary shoot during a season. As one ovule aborts
development,
the primary shoot grows two more ovular shoots, one of which again
aborts; this repeatedly occurring four more times
before cessation of growth
results in an axillary shoot with five ovules. |
|
|
|
Fig. 11. Taxus brevifolia var. polychaeta, Photo by Peter Baye (Sep 2010), same location as Fig. 10. Shows two arillocarpia on a primary shoot and two of the supposedly three pairs of decussate scales for each ovular secondary shoot. Thus, the secondary shoot in this variety appears relatively short. |
|
Plants of Taxus brevifolia var. polychaeta—that frequently develop two subterminal ovules—may also continue to grow a new secondary shoot where one of the two ovules aborts development, instead of terminating growth with a single ovule. This may repeatedly occur a number of times in a leaf-axillary shoot complex. Consequently, ovules on the lower portion of the shoot complex then appear lateral as observed in plants from Sonoma County, California (Fig. 10). This is not the same as multiple secondary shoots arising in the axils of different scales of a primary shoot as shown by André (1956) and Anderson (2001), which can occur over a period of several seasons (Dupler 1920; André 1956). The typical cone of a modern conifer is a more specialized compound structure having a determinate growth consisting of a central shoot axis with spirally (or decussate) arranged ovuliferous scales and bracts, each fertile (ovuliferous) scale subtended by a bract, in which the two are variously united or separated among the genera. Fleshy scales—not necessarily aril-like—are found in families such as the Austrotaxaceae, Amentotaxaceae, Cephalotaxaceae, Cupressaceae, and Podocarpaceae. The family Taxaceae, as interpreted here, includes only one other genus, Pseudotaxus; its ovuliferous organ consists of only a primary shoot with a white instead of red aril (Florin 1948). Rudolf Florin, known for his extensive studies on gymnosperms, promoted the classification of “taxads” (includes extant genera Austrotaxus, Amentotaxus, Torreya), in a separate class (Taxopsida, Florin 1958) by emphasizing the terminal position of a single ovule developing as a continuation of the shoot axis itself as he illustrated in the following figure (Fig. 12). |
|
|
|
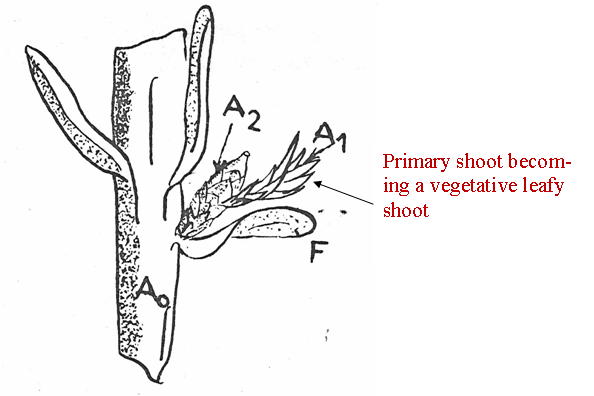
Fig. 12A-B. Comparison of illustration from Florin (1951, his Fig. 65 and caption), here referred to as Fig. 12A, with that from André (1956, his Fig. 39), here referred to as Fig. 12B. Illustration from André (12B) shows three secondary shoots developing on a primary shoot over a period of three different seasons. A1 = primary shoot (clear). A2 = Secondary shoot (stippled). F = Spirally arranged bracts, terminology according to André (1956) which he referred to as scarious leaves, in contrast to decussate arranged scales (André's term) on the secondary shoot. Not shown in this particular illustration, but in another illustration by André, are two pairs of transverse scales at the base of the primary shoot. ar = aril, ov = ovule. |
|
Nevertheless, André (1956) presented a schematic structure (Fig. 12) that would seem to indicate that the Taxus cone is not all that different from the conifer cone. Indeed, the ovuliferous scale in the conifer cone may correspond to the secondary shoot axis of Taxus as suggested by Miller (1988), and the conifer bract equivalent would then be the subtending scale on the primary shoot. The ovule in Taxus has also been viewed as a separate terminal shoot (Stützel & Röwekamp 1999). However, it should be noted that the primary shoot of Taxus may continue to reproduce secondary shoots in subsequent seasons where they had fallen off in previous seasons, or primary shoots may remain dormant for one or more seasons, or they may develop into a vegetative leafy shoot and eventually become a branch not unlike the other branches (Strasburger 1889; Robertson 1907; Dupler 1920; André 1956, Fig. 38), Fig. 12. |
  |
|
Many botanists emphasize the absence of a bract at the base of the secondary shoot and/or the absence of an ovuliferous scale in Taxus to justify that it does not bear cones as generally recognized in conifers (Florin 1951; Foster & Gifford 1974; Cope 1998). However, “functional attributes of cone structures need to be considered in a very broad context” (Tomlinson and Takaso 2002); thus, there are Taxus cones, Pinus cones, phylloclad cones, podocarp cones, etc. Seed cones of Cupressaceae, which have been compared to Taxaceae (André 1956), are thought to have evolved by increasing loss of brachyblasts in the lower (proximal) region, with ovules developing only in the distal region in Cupressus and Juniperus, in which the latter genus also has cauline ovules (André 1956; Schulz et al. 2003). The discovery of multiovular branched shoots in Sonoma County plants of Taxus brevifolia var. polychaeta (worm cone yew) during June 2010 has led Spjut to briefly look for this variety in Washington and northern Idaho in conjunction with other field work. Worm cone yew could not be found in the vicinity of the type locality near Olympia, WA (Mud Bay), where undoubtedly urban expansion has reduced its habitat since its collection from there in 1938. A review of Taxus specimens at the Stillinger Herbarium (Moscow, ID) was also negative in regard to a specimen sent to Spjut in 1992 by the USDA Forest Service from Coeur d'Alene. These few specimens known so far of var. polychaeta from Washington and Idaho have relatively long unbranched ovuliferous shoots, 5–8 mm long, compared to less than 5 mm in length for plants in Sonoma County, California. The ovulate shoots of the Sonoma County plants appear distinctive for their relatively short secondary branched shoots that result from where one of two subterminal ovules aborts in development. Variety polychaeta may also be expected in Mendocino County. Additionally, the multi-ovulate shoots of Taxus generally thought to be rare in the genus are surprisingly quite frequent in T. brevifolia vars. brevifolia and reptaneta, perhaps in 20% of the female individuals or genets. Problems in studying reproductive shoots of Taxus in the field involve not being able to reach the branches of trees, or only the lower branches are accessible, or the plants are not easily accessible because they grow on steep slippery slopes. Another problem unrelated to access is that in T. brevifolia var. reptaneta, male genets (clones) are often more common than female genets. Differences in ovular shoot development should be expected in other species of Taxus as suggested for the developmental differences reported between T. recurvata and T. baccata, and the paired arillocarpia commonly seen in T. baccata var. dovastoniana (Spjut 2007b).
In descriptions of Taxus
species on the web pages of worldbotanical.com., the age of the branch having
arillocarpia was determined by counting the successive sections of branchlets
with persistent scales; however, the age of the primary shoot could be older
than indicated. Most species of Taxus produce arillocarpia on
second
year or older branches. An exception is the East Asian T. biternata
that has arillocarpia on the current season growth. Other yew species in
temperate East Asia show notable differences in seed shape and in layering (see
Introduction to Taxonomy of Taxus).
A lot more field work needs to be done on Taxus to better understand
the species taxonomy and reproductive biology. |
|
Literature Cited André, D. 1956. Contribution à l’étude morphologique du cône femelle de quelques gymnospmermes (Cephalotaxacées, Juniperoidées, Taxacées). Nat. Monspliensia (Bot.) 8: 3–35. Anderson, E. D. 2001. Reproductive biology of the Pacific yew (Taxus brevifolia). University of Victoria. National Library of Canada, Ottawa, Ontario. Anonymous. 1913. Kew Bull. 1913: 255–263. Bertoloni, A. 1862. Miscellanea botanica XXIII. Mem. Acad. Sci. sér 2: .228–229, tab. Bobrov, A. V. F. CH., A. P. Melikian and E. Y. Yembaturova. 1999. Seed morphology, anatomy and ultrastructure of Phyllocladus L. C. & A. Rich. ex Mirb. (Phyllocladaceae (Pilg.) Bessey) in connection with the generic system and phylogeny. Annals Bot. 83: 601–618. Chadwick, L. C. and R. A. Keen. 1976. A study of the genus Taxus. Ohio Agric. Res. Bull. 1086. Chamberlain, C. J. 1935. Gymnosperms, structure and evolution, University of Chicago Press, Illinois. Cheng (Zheng), W-c., L-k. Fu, and C-y. Cheng. 1975. Gymnospermae Sinicae. Acta Phytotax. Sin. 13(4): 56–90 & 66 illus., 17 plates. Cheng (Zheng) W-c., and L-k. Fu. 1978. Taxaceae. In: Fl. Reipub. Pop. Sin. 7, Gymnospermae. Agendae Academiae Sinicae. [English Translation]. Cheng, Y-p., S-y. Hwang and T-p. Lin. 2005. Potential refugia in Taiwan revealed by the phylogeographical study of Castanopsis carlesii Hayata (Fagaceae). Molecular Ecol. 14: 2075–2085. Collins, D., R. R. Mill, and M. Möller. 2003. Species separation of Taxus baccata, T. canadensis, and T. cuspidata (Taxaceae) and origins of their reputed hybrids inferred from RAPD and cpDNA data. Amer. J. Bot. 90: 175–182. Cope, E. A. 1998. Taxaceae: The genera and cultivated species. Bot. Rev. 64: 291–322. Croom, E. M., Jr. 1995. Taxus for taxol and taxoids. In M. S. Suffness, ed., Taxol. Science and applications. CRC Press. Pp. 37-70. Dupler, A. 1919. Staminate strobilus of Taxus canadensis. Bot. Gaz. 68: 3456–366. Dupler, A. 1920. Ovuliferous structures of Taxus canadensis. Bot. Gaz. 69: 492–520. [Available online-Google Books]. Earle. C. J. 2010. The gymnosperm database. http://www.conifers.org. Eckenwalder, J. E. 2009. Conifers of the world. Timber Press, Portland, OR. Esselstyn, J. A. and C. H. Oliveros. 2010. Colonization of the Philippines from Taiwan: a multi-locus test of the biogeographic and phylogenetic relationships of isolated populations of shrews. J. Biogeogr. 37:1504–1514. Farjon, A. 1998. World checklist and bibliography of conifers. The Royal Botanic Gardens, Kew. Farjon, A. 2001. World Checklist and Bibliography of Conifers. 2nd edition. The Bath Press, Bath, United Kingdom. Ferguson, D. K. 1978. Some current research on fossil and recent taxads. Rev. Palaeobot. Palynol. 26: 213–226. Flora of China. Vol. 4. Cycadaceae to Fagaceae. Missouri Botanical Garden, Science Press, Beijing. Florin, R. 1948. On the morphology and relationships of the Taxaceae. Bot. Gaz. 110: 31–39. Florin, R. 1951. Evolution in cordaites and conifers. Acta Horti Berg. 16: 285–388, plate.
Florin, R. 1958. On Jurassic taxads and conifers from north-western Europe and eastern Greenland. Acta Horti Berg. 17: 257–402, 56 plates.
Foster, A. S. and E. M. Gifford, Jr. 1974. Comparative morphology of vascular plants. 2nd ed. W. H. Freeman and Company, San Francisco. Fu, L-k, N. Li, and R. R. Mill. 1999. Taxaceae. Fl. China 4: 89–96, Missouri Botanical Garden Press, St. Louis. Goodman, J. and V. Walsh. 2001. The story of taxol. Nature and politics in pursuit of an anticancer drug. Cambridge University Press. Greuter, W., J. McNeill, F. R. Barrie, H. M. Burdet, V. Demoulin, T. S. Filgueiras, D. Nicolson, P. C. Silva, J. E. Skog, P. Trehane, N. J. Turland, and D. L. Hawksworth. 2000. International code of botanical nomenclature (Saint Louis Code) adopted by the 16th International Botanical Congress. St. Louis, MO, July–August 1999. Koeltz Scientific Books, Königstein, Germany. {Note: A later update with few changes was published; however, the author of this web page no longer supports the IAPT because of their hypocritical practice]. Griffith. W. 1847-1848. Journals of Travels in Assam Burma Bootan Affghanistan and the Neighbouring Countries (sic). Vol. II. Itinerary notes of plants collected in the Khasyah and Bootan Mountains. Bishop's College Press: Calcutta. Munshiram Manoharlal Publishers, New Delhi). Griffith, W. 1854. Notulae ad plantas asiaticas. Posthumous papers bequeathed to the Honorable The East India Company, arranged by John M’Clelland, Bishops’ College Press, Calcutta. Vol. 4. Hageneder, F. 2007. Yew. A history. Sutton Publishing Ltd., Thrupp-Stroud-Goucestershire. Hao, D-c., B-l Huang and L. Yang. 2008a. Phylogenetic relationships of the genus Taxus inferred from chloroplast intergenic spacer an nuclear coding DNA. Biol. Pharm. Bull. 31: 260–265. Hao, D-c., P-g. Xiao, B. Huang, B-g. Ge and L. Yang. 2008b. Interspecific relationships and origins of Taxus and Cephalotaxus revealed by partitioned Bayesian analyses of chloroplast and nuclear DNA sequences. Plant Syst. Evol. 276: 89–104. Harris, T. M. 1976a. Two neglected aspects of fossil conifers. Amer. J. Bot. 63:902–910. Harris, T. M. 1976b. The Mesozoic gymnosperms. Rev. Paleobot. and Palyn. 21:119–134. Hickman, J. C. The Jepson Manual. Higher plants of California. University of California Press, Berkeley. Hils, M. 1993. Taxaceae Gray. Yew family. Fl. North America 2: 423–427. Hooker, J. D. 1890. Flora of British India. London: L. Reeve & Co. Hu, S-y. 1964. [Notes on] the Flora of China. Taiwania: 13–62. International yew resource conference. 1993. Yew (Taxus) conservation biology and interactions. Berkeley, CA. Jarvis, C. E., F. R. Barrie, D. M. Allan, and J. L. Reveal. 1993. A list of Linnaean generic names and their types. Regnum Veg. 127. Jepson, W. L. 1939. A flora of California. Vol. 3 (Pt. 1). University of California, Berkeley. Kvaček, Z. 1984. Tertiary taxads of NW Bohemia. 1982 Acta Univ. Carol., Geol., Pokorny 4: 471–491. Laubenfels, D. J., de. 1953. The external morphology of coniferous leaves. Phytomorphology 3:1–20. Laubenfels, D. J., de. 1978. The taxonomy of Philippine Coniferae and Taxaceae. Kalikasan 7: 117–152. Laubenfels, D. J., de. 1988. Coniferales. Fl. Malesiana 10(3): 337–453. Li, J., C. C. Davis, P. Del Tredici, and M. J. Donoghue. 2001. Phylogeny and biogeography of Taxus (Taxaceae) inferred from sequences of the internal transcribed spacer region of nuclear ribosomal DNA. Harv. Pap. Bot. 6: 267–274. Linnaeus, C. 1753. Species plantarum. Laurentii Salvii, Stockholm. [Note: Linnaeus also included Taxus nucifera. This was later discovered by Siebold and Zuccarini (1843) to belong to the genus Torreya]. Lu, S-y., C-l. Peng, Y-p. Cheng and K-h. Hong. 2001. Chloroplast DNA phylogeography of Cunninghamia. Genome 44: 797–807. Marshall, H. 1785. Arbustum Americanum. Joseph Crukshank, Philadelphia. Miller, C. N., Jr. 1977. Mesozoic conifers. Bot. Rev. 43: 217–280. Miller, C. N., Jr. 1988. The origin of modern conifer families. In C. B. Beck, ed., Origin and evolution of gymnosperms. Columbia Univ. Press, New York. Pp. 448–486. Möller, M., L-m. Gao, R. R. Mill, D-z. Li, M. L. Hollingsworth, and M. Gibby. 2007. Morphometric analysis of the Taxus wallichiana complex (Taxaceae) based on herbarium material. Bot. J. Linn. Soc. 155: 307–335. Munz, P. and D. D. Keck. 1959. A California flora. University of California Press. Natural Resources Ecology GIS Database in Taiwan, online. Accessed Nov 2010. http://econgis.forest.gov.tw/rareplant/species; in Chinese; use key word search: "Taxus Taiwan econgis" followed by Google Translate (for English). Pant, D. D. 2000. Inclusion of Taxaceae in a separate order, Taxales. Current Sci. 79: 278–279. Parker, V. T., M. C Vasey and J. E. Keeley. 2009. Arctostaphylos. Flora North America Vol. 8: 406–444. Perdue, R. E., Jr. and J. L. Hartwell. 1969. The search for plant sources of anticancer drugs. Morris Arb. Bull. 20(3): 35–53. Pilger, R. 1903. Taxaceae-Taxoideae—Taxeae. Taxus. In Engler, Das Pflanzenreich IV: 110–116. Pilger, R. 1909. Die Morphologie des weiblichen Blüten Sprösschens von Taxus. Bot. Jarb. 42: 241–250. Pilger, R. 1916. Die Taxales. Mitt. Deutsch. Dendrol. Ges. 25: 1–28. Pilger, R. 1926. Taxaceae. In Engler, A. and K. Prantl (eds.), Die natürlichen Pflanzenfamilien, 2nd ed., 13: 199–211. Quinn, C. J., R. A., Price and P. A. Gadek. 2002. Familial concepts and relationships in the conifers based on rbcL and matK sequence comparisons. Kew Bull.: 57: 513–531. Rehder, A. 1923. New species, varieties and combinations from the herbarium and the collections of the Arnold Arboretum. Taxus media hybr. nov., Taxus media f. hatfieldii forma nov., Taxus media f. hicksii, comb. nov. J. Arn. Arb. 4: 107–109. Rehder, A. 1940. Manual of cultivated trees and shrubs (2nd ed.). MacMillan Co., NY. Robertson, A. 1907. The Taxoideae: A phylogenetic study. New Phytologist 6: 92–102 and Plate. Schulz, C., A. Jagel and T. Stützel. 2003. Cone morphology in Juniperus in the light of cone evolution in Cupressaceae s.l. Flora 198: 161–177. Semikhov, V.F., L. P. Aref'eva, O.A. Novozhilova, A. S. Timoshchenko, and D. S. Kostrikin. 2001. Systematic relationships of Podocarpales, Cephalotaxales, and Taxales based on comparative seed anatomy and biochemistry data. Biol. Bull. Russian Acad. Sci. 28: 459–70. Shah A, D-z Li, M. Möller, L-m Gao, M. L. Hollingsworth and M. Gibby. 2008. Delimitation of Taxus fuana Nan Li & R. R. Mill (Taxaceae) based on morphological and molecular data. Taxon 57: 1–12. Shah, A., L-m. Gao, D-z Li, and M. Möller. 2010. Species Delimitation and Conservation Genetics of Taxus fuana Nan Li & R.R. Mill (Taxaceae) in Pakistan. III International Workshop, Mar 25-26, Ponferrada, León, Spain. Abstract. Siebold, P. F., de and J. G. Zuccarini. 1843. Plantarum, quas in Japonia collegit Dr, Ph. Fr. de Siebold. Genera Nova. Notis characteristicis delineatiionibusque illustrata proponunt, I. Abh. Math.-Phys. Cl. Königl. Bayer. Akad. Wiss. 3. Silba, J. 1984. An international census of the coniferae, I. Phytologia Mem. 7: 1–79. Silba, J. 1986. Encylopaedia coniferae. Phytologia Mem. 8: 1–217. Spjut, R. W. 1977. [USDA, ARS Memorandum, July 14]. Taxus brevifolia (Taxaceae) reviewed by GMC (Gudrun M. Christensen). A review of literature on taxonomy, ecology, and geographical distribution of the species, including its description, summary of geographical distribution by state or province, and literature reviewed. Distributed periodically during 1981-1992 by the National Cancer Institute to prospective suppliers, without reference to the author, for solicitations of contract bids on up to 30 tons of bark. Spjut, R. W. 1992. A taxonomic key to the species of Taxus. NCI Workshop on Taxus, Taxol, and Taxotere, Rockville, MD (Abstract only). Spjut, R. W. 1993. Reliable morphological characters for distinguishing species of Taxus (Abstract). In International yew resource conference. Yew (Taxus) conservation biology and interactions. Berkeley, CA. Pp. 39–40. Spjut, R. W. 1994. A systematic treatment of fruit types. Mem. New York Bot. Gard., Vol 70.
Spjut, R. W. 1998. Two papers
presented at the AIBS Annual Meeting, American Systematic Plant Taxonomists,
Baltimore Convention Center, MD, 5 Aug. 1998. Abstracts published on the
Internet, Botanical Society of America. Spjut, R. W. 2000. Three papers presented at the joint meetings of the Botanical Society of America and American Systematic Plant Taxonomists, Portland, OR, August, abstracts published online and Amer. J. Bot. (a)
A
phytogeographical analysis and classification of leaf morphological features in Taxus
(Taxaceae).
http://www.ou.edu/cas/botany-micro/botany2000/section13/abstracts/27.shtml.
Spjut, R. W. 2003. Nomenclatural and taxonomic review of three species and two varieties of Taxus (Taxaceae) in Asia. www.worldbotanical.com (Prepared 1998, submitted to Sida in 2000, accepted for J. Bot Res. Inst. Texas in 2006). Spjut, R. W. 2006. Biogeographical data on putative hybrids for species of Taxus (Taxaceae) in the Himalayas and North America. Abstract, Botanical Society of America, Chico, CA, July 29–Aug 2. Spjut, R. W. 2007a. A phytogeographical analysis of Taxus (Taxaceae) based on leaf anatomical characters. J. Bot. Res. Inst. Texas 1(1): 291–332. Spjut, R. W. 2007b. Taxonomy and nomenclature of Taxus (Taxaceae). J. Bot. Res. Inst. Texas 1(1): 203–289. Spjut, R. W. 2010. A revised taxonomic key to the species and varieties of Taxus (Taxaceae). III International Workshop, Poster, Mar 25-26, Ponferrada, León, Spain (http://www.amorteira.org/PDF/yew%20workshop_secondcall.pdf). Submitted as three pdf files. Strasburger, E. 1889. Handbook of practical botany. 2nd Ed. MacMillan & Co., London. Stützel, T. & I. Röwekamp. 1999. Female reproductive structures in Taxales. Flora (Jena) 194: 145 – 157. Thomas, B. A. and R. S. Spicer. 1987. The evolution and palaeobiology of land plants. Vol. 2. Croom Helm, London and Dioscorides Press, Portland. Tomlinson , P. B. and T. Takaso. 2002. Seed cone structure in conifers in relation to development and pollination: a biological approach. Can. J. Bot. 80: 1250–1273. USDA Agricultural Research Service, Germplasm Resources Information Network (GRIN). Taxonomic queries—Taxus. Last update reported, 27 Dec 2005. Last accessed Nov. 2010. Wallich, N. 1826. Tentamen florae nepalensis illustratae. Fasc. 2. Calcutta and Serampore. Wani, M.C., H. L, Taylor, M. E. Wall, P. Coggon and A. T. McPhail. 1971. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Amer. Chem. Soc. 93: 2325–2327. Wilson, C. L. 2005. The telome theory. Bot. Rev. 71: 485–505. Yu, H-t. 1995. Patterns of diversification and genetic population structure of small mammals in Taiwan. Biol. J. Linn. Soc. 55: 69–89. Zhang, X-m., L-m. Gao, M. Möller, and D-z Li. 2009. Molecular evidence for fragmentation among populations of Taxus wallichiana var. mairei, a highly endangered conifer in China. Can. J. For. Res. 39: 755–764.
|