Taxus celebica from Yunnan, Forrest s.n. (A) |

Taxus celebica from Tibet (“Assam”), Rima, 7000 ft., Kingdon
Ward 19324 (BM) |
|
 |
| |
12.
Taxus celebica (Warb.) H. L. Li (Figs.3B, 23), Woody Fl. Taiwan
34. 1963. Cephalotaxus celebica Warburg, Monsun. I: 194. 1900,
one specimen cited—Holotype: INDONESIA. Sulawesi (Celebes): southern, Gipfel des
Wawo-Kraeng, on the forest-clad summit, 2800 m, Warburg 16889 S
(photocopy!), fragment of type at B that was destroyed.
Warburg
distinguished C. celebica from C. sumatrana, and excluded
Podocarpus celebicus Hemsl.
Podocarpus celebicus
Hemsley, Kew Bull. 39. 1896. TYPE: INDONESIA. Sulawesi
(Celebes): southern, Bonthain Peak, 7000–10,000 ft [Sep. 1895]. A. H. Everett 35 (holotype:
K!).
Celebes Yew. Distribution:
Forest margins, 1150–3100 m; Nepal, Bhutan, NE India, South Vietnam,
China (Tibet, Yunnan, Sichuan),
Indonesia (Sulawesi).
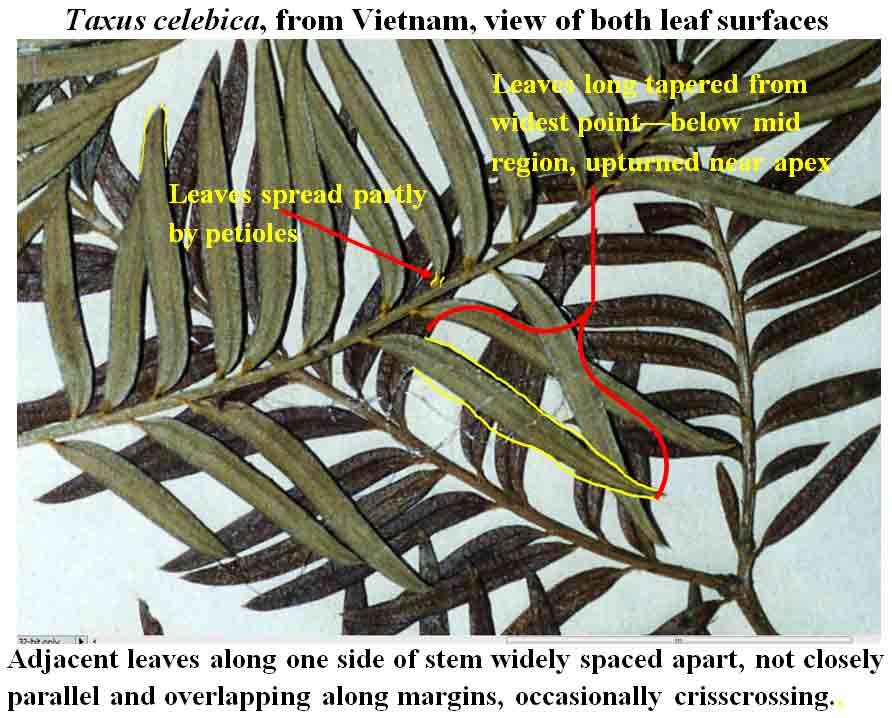
Tree 1–20 m high; branchlets yellowish-green to yellowish-red;
bud-scales partly persistent, 3-seriate, loosely adnate, minute deltoid,
concave and shortly cuspidate, longest ones 0.5–1 mm long, glossy
brown, lower carinate, upper smooth, firm. Leaves persistent on older
twigs, spreading in two ranks from decurrent petiole 8–20 mm long, not
overlapping, nearly parallel, narrowly lanceolate, slightly falcate,
2.5–4 cm long, 3–5 mm wide at widest part of leaf, relatively
thinner than most other species, 175–300 µm thick, light green and
plane above to a slight, abruptly elevated midrib, pale green and plane
below to a flush to slightly rounded midrib, the abaxial marginal region
usually (18-) 27–36 cells wide, abruptly differentiated from stomata
bands, often not markedly discolored upon drying, mostly with long
rectangular epidermal cells, these (3-) 5–15× l/w, thin-walled, not
inflated, entirely epapillose, or papillose to one-half of the marginal
region; abaxial midrib cells similar, mostly without papillae, or
papillose on outer 2–3 rows; papillae medial to submarginal; stomata
bands yellowish to distinctively whitish, narrower than the broad
marginal and midrib regions; stomata monocyclic, in 11–22 distinct
rows/band, each row separated by 1–2 rows of accessory trapezoidal
cells with small papillae; upper (adaxial) epidermal cells in transverse
section elliptical, ca. 20 µm tall and 25 µm wide, thin-walled; lower
(abaxial) epidermal cells not as large, 10–20 µm tall, 20–40 µm
wide. Palisade parenchyma 1 row, often short, or indistinct (0–30 µm
long). Male cones not seen. Female cones in one specimen plentiful; seed
conical, greenish with a dark brown umbo.
Taxus celebica is
recognized by the relatively large, glossy, pale green
leaves that have a lanceolate shape, a flattened surface, plane
margins (not revolute), relatively narrow stomata bands in contrast to a broad marginal zone of long
rectangular cells, and by other leaf epidermal features as seen in specimens
from Sulawesi (type), Yunnan (Forrest 7798), Sichuan (H. Smith
10401, Plate 6 in Florin 1948a), South Vietnam (Schmind s.n.),
Bhutan (Cooper & Bulley 2833), Khasia (Clarke 38308)
and Tibet (Kingdon Ward 19324).
The
leaf stomata bands
of T. celebica have 8–12 (-14) rows of stomata compared to
(11-) 14–19 (-21) stomata rows in T. mairei.
The accessory cells are often distinct from other species in the genus by their
short wedge shape (<3× l/w) and by the relatively small
(minute) medial papillae that are more distant from one another.
The stomata bands are further differentiated by the marginal
zone of long rectangular
cells (>10× l/w), mostly without papillae across (13-) 24–36 cells, in contrast to the oblong leaves with shorter trapezoidal
cells of T. mairei, or the transitional zone of
papillose cells that is usually seen in the Baccata and Wallichiana
Groups.
These
features of T. celebica suggest a separate
evolutionarily line (Sumatrana Group) in which leaf stomata and papillae
have become reduced as a result of adapting to warmer
climates at lower elevations in tropical areas.
This is undoubtedly related to differentiation of the
stomata bands by color—that is clearly evident to the naked eye by the
contrast of the reddish discoloration in the marginal and midrib regions
(dried leaves), especially T. mairei and T. sumatrana.
However, one may also argue that
T.
celebica, along with T. mairei, should
be included under T. sumatrana due to various intermediates that
are difficult to assign to any species in this group. My previous annotations of
T. celebica were based on
leaves having most of the following features: long epidermal cells, obscure palisade
layer of parenchyma cells, and flattened shape in T-section, but I have
since decided that taxonomic weight should be given to the lanceolate
acuminate type of leaf. This also led to distinguishing T.
sumatrana by the puckered leaf in dried specimens.
An example of a variant of
T. mairei that I had earlier considered T
celebica differs from T.
celebica only by the leaves tapering to an acute or obtuse apex,
instead of an acuminate apex—as seen in
specimens from Fujian (Price 1258b), Guizhou (Steward et al.
328), Sichuan (Wang 20541), Guangdong (Nanling Expedition
1838), and Ningxia Huizu (Chao 1223).
These specimens belong to var. speciosa, distinguished by the
relatively large leaves that are green in dried specimens. Another
example of T. mairei has the acuminate leaves of T. celebica
(especially young or immature leaves) but differs by their more
elliptical instead of lanceolate shape, and by their abaxial midribs
having mammillose epidermal cells. This includes duplicates of the
original material for T. mairei from Yunnan (Maire
s.n.) and additional specimens from Taiwan (C-j. Chang, from
Hua-lien).
The mammillose epidermal cells as seen in leaf T-sections,
often accompanied by a layer of enlarged parenchyma cells lying closely
against the midrib epidermal cells, is characteristic of T. mairei.
Most other specimens of T. mairei further differ by thicker
leaves that are more convex across the adaxial surface, and less evenly
tapered to an obtuse apex. The characteristic leaf anatomical
features apply to both var. mairei and var. speciosa.
The
leaf anatomical features of long epidermal cells, a broad marginal zone
of epapillose cells, and short wedge-shaped accessory cells with minute
papillae, have been observed in some T. mairei specimens where
cell length and development of papillae may also vary on leaves from the
same plants. For example, a
specimen cultivated at Kew Gardens has leaves shaped like T. mairei
with stomata bands similar to T. celebica (wedge shaped cells,
small papillae, yellowish chloroplast), while a blind study of numerous
leaves from a related specimen in cultivation (Phyton s.n.), and
also from Taiwan (Hua-lien, Chang 1–5,7-10), the leaf epidermal
cells varied in length and in shape in which young leaves were
determined to be T. celebica or T. sumatrana. This
suggests that T. mairei may have been derived from an ancestral T.
sumatrana complex, and it may have also
subsequently hybridized with this species—giving rise to many
combinations between leaf shape and the anatomical features.
Although development of papillae in the leaf marginal zone is
variable within the Sumatrana Group (Appendix), the papillose
marginal cells can be easily distinguished from those of the stomata
bands. I interpret this as
evidence of papillae having evolved secondarily in the group,
consequently, more influenced by environmental factors.
Taxus sumatrana
var. atrovirens is very similar to T. celebica in having
relatively large leaves that appear green in dried specimens. It
is distinguished by the puckered leaf, appearing strongly channeled on
the adaxial surface along the midrib. Specimens from the
Arnold Arboretum Herbarium under this variety were previously annotated
T. celebica. While this variety appears intermediate to
T. celebica, the character feature of the puckered leaf helps tie
together T. sumatrana and its varieties, including var.
concolorata. There is the temptation to treat all species as
subspecies in the Sumatrana Group under T. sumatrana;
however, classifications with subspecies and varieties of a species are
in my opinion less useful. Another alternative is to treat T.
celebica as a variety of T. sumatrana, as I have temporarily
indicated on this page, but my preference is to maintain this taxon as a
distinct species. On the other hand, because specimens from Nepal
and Bhutan appear to intergrade with T. kingstonii, another
solution was to treat them as a distinct species for which an epithet
was chosen based on the acuminate leaf as indicated in an
unpublished key included in a research proposal (April 1995).
Representive Specimens—Nepal Harain Letty, 16 Oct. 1902 (BM), 17 Oct 1802 (GH,
p.p., left specimen). Bhutan: illegible, 9,000-10,000 ft, Griffith
2006 (K, p.p., with T. wallichiana). India—Khasi
Hills, “Mawphlang,” scattered in the evergreen Sacred Grove, 6000
ft, erect tree, Kingdon-Ward 18751 (A); Khasia (Hills), “Maophlang,”
Clarke 38308 (K); presumably collected in Khasi Hills, locality
illegible, 10 ft high, label from Kew Herb. Hooker, in adnot.
Cephalotaxus mannii (GH); Khasi Hills, Nungluai, 6,000 ft, G.
Mann s.n. (A, P). South Vietnam: Da Lat: ravin buisé an
chalet Rimaud, Evrard 305 (P: 2 sheets); Langfian, Evrard 1438
(P); Da Lat: Dan Tria, Manline, 1400 m, 1610 m, Schmid, yr 1960
(P); same location, Cuong 1289 (P); Dua Lamghi, Schmid, yr
1960 (P). China—Tibet: “Assam,” Rima, 7000 ft., Kingdon
Ward 19324 (BM). Yunnan: Forrest 11789 (K); Forrest
s.n. (A). Sichuan: W region, between Huangnipu and Yaan (Yachou),
Malingtsang, 900 m, H. Smith 10401
(A, BM); vicinity of Wah-hsien, Mou-tao-chi, Metasequoia area,
Li-chuan, Jian-Nan-Hsien, Ta-pen-Ying, 3800 ft, in forest, tree, C.T.
Hwa 027 (A, K).
|