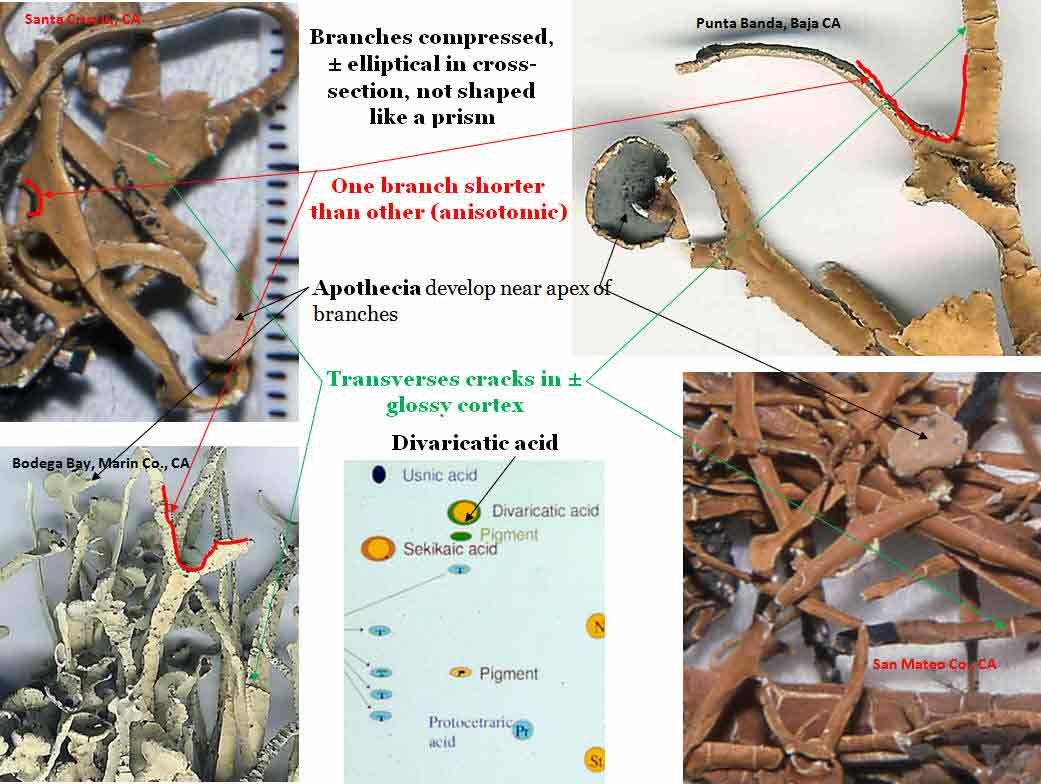
Niebla homalea
(Acharius) Rundel & Bowler
is a fruticose lichen first described by Acharius in 1810 as a species of Ramalina from
specimens collected on rocks in California, most likely
near Point Reyes in Marin County. The species is characterized
by a thallus containing divaricatic acid (with triterpenes) and by divided into
narrow
±cylindrical branches, which occasionally divide into
secondary branches of unequal length. The branch margins frequently twist 90°.
The cortex is usually glossy and transversely cracked at various intervals.
Niebla homalea is widely distributed from Point Navarro in
Mendocino County, California, to Isla Guadalupe and
slopes leading up to Mesa Camacho in the northern half of Baja California
peninsula.
A 6-loci phylogenetic tree of Niebla homalea (Spjut et
al. 2020) shows the species in
a large clade sister to N. turgida collected near San Antonio del
Mar. The California and Baja California specimens belong to
separate sister subclades and both include N. eburnea, which also
occurs elsewhere in the phylogenetic tree. The Baja California
subclades include other species identifications such as N.
testudinaria, N. juncosa var. spinulifera and N.
flagelliforma. These subclades represent separate cryptic species complexes in
California and in Baja California. Niebla homalea in a strict
sense may prove
endemic to Marin County; the putative type is from Point Reyes. BPP analysis delimited three species.
Most specimens were found associated with N. eburnea.
Jorna et al. (2021) reported they prepared a draft genome from a
specimen representing N. homalea collected from Sonoma
Coast State Park, Shell Beach. This draft has been published (Ametrano
in Duong et al. 2021).
The Niebla
homalea species complex is not the only example in which thalli
found growing in close proximity to one another show up in different
genomic clades as shown above for Niebla homalea specimens
collected on the Pt. Reyes Peninsula. This is commonly seen in
many if not most phenotypic species. Examples in the depside group
include
N. contorta,
N. dilatata,
N.
lobulata,
N. rugosa
and others. These complexes may be regarded to consist of cryptic
species, and/or in other cases cryptic hybrids where phenotypic
differences are evident in the sister clades. This may involve variation
in chemotypes such as seen with
N. effusa
and N.
palmeri. Hybridization seems the best explanation for
hybrid chemotypes between
N.
spatulata and N. contorta, especially where fusion
of thalli are evident.
In 1852, Montagne recognized Ramalina homalea
as belonging to a new genus he named
Desmazieria. Unfortunately,
this was similar to “Desmazeria” named earlier by Dumortier 1822 for a genus of grasses.
Although lichens have little in common with grasses, the
International Code of Nomenclature (ICN) allows only one
name, the later homonym by Montagne must be rejected unless conserved. Follmann (1976),
who apparently
discovered Dumortier's earlier name, stated that the names for
the two genera “do not sound sufficiently similar that they are likely
to be confused. Therefore, the genus name Desmazieria Mont.
can be retained.” However, Rundel and Bowler (1978) disagreed;
they created the substitute name Niebla.
Montagne's name for the one species was
clear, “Desmzieria
homalea Montag.” His description of the species
was followed by reference to synonyms that
included not only his Usnea ceruchis, but earlier synonyms “Borrera
ceruchis Ach.?” and “Ramalina ceruchis” De Notaris (1846). Since Montagne (1852)
recognized only one species, the type has to be Desmazieria
homalea (Acharius) Montagne 1852 (ICBN Art. 7.1–7.4), which is now Niebla homalea (Acharius) Rundel & Bowler
1978.
Subsequently, Rundel and Bowler (in Rundel et al. 1972), recognized two
additional species under the illegitimate Desmazieria,
Niebla sensu Spjut (1995, 1996), later legitimized in Niebla
(Rundel & Bowler 1978), N. josecuervoi and N. pulchribarbara
from thalli growing around Bahía
de San Quintín,
Baja California. However, their 'beautiful' lichen
N.
pulchribarbara was subsumed under
N. josecuervoi, (Bowler and
Marsh 2004) in further honor of their field assistant who the lichen was
named after. Thus, only three North American species of Niebla sensu Spjut were
recognized by Bowler and Marsh (2004; N. homalea,
N.
isidiaescens, N. josecuervoi).
It
is interesting to note that Vermilacinia laevigata (Niebla
laevigata, Bowler et al. 1994) had been overlooked
for many years because of its superficial resemblance to N. homalea. Mason Hale's
(Hale & Cole 1988) Lichens of California included a color photograph of Vermilacinia laevigata referred to as N. homalea despite knowing that it might
potentially be V. halei Spjut (unpublished) from his review of Spjut's manuscript and
numerous specimens at the Smithsonian Institution with this name,
and from Spjut's review of his manuscript for which
he remarked that his Niebla names were probably not correct.
Unfortunately, V. halei Spjut in editus had to be retracted from publication, because Bowler et
al. (1994) published their name (N. laevigata) as Spjut (1996) was in
press.
Howe (1913), who monographed the North American Ramalina, was
obviously aware of the morphological differences between Vermilacinia
and Niebla—by his remark that he considered the chondroid
strands of Ramalina (Niebla) “almost of generic
importance”—and perhaps would have treated them in different genera had
he had the chemical tools of modern lichenology such as microcrystal
tests (1930's–) and thin-layer-chromatography (TLC, 1950's–).
Additionally, it appears that misidentification of an unknown triterpene
(T3, Spjut 1996), referred to as methyl 3,5 dichlorolecanorate by Rundel
& Bowler on their chemical annotation labels of specimens at the US
(Smithsonian Institution, herbarium), and the lack of clear reference to
the key chemotaxonomic diterpene in Vermilacinia tuberculata by Riefner
et al. (1995, as Niebla tuberculata), which was not clearly
distinguished from zeorin, may
explain their inability to accept Vermilacinia (Spjut 1995).
Another problem is that chemical differences in these lichens are not
distinguishable by chemical spot tests, introduced by Nylander during
the 1860's (Molnára & Farkas 2010) at which time lichenologists
considered lichen species based on chemical differences nonsense,
although some mycologists today, who review NSF grants, still hold that
view
Triterpenoids in Niebla homalea include novel stictanes, named
nieblastictanes and nieblaflavicanes (Zhang
et al. 2020; Diaz-Allen et al. 2021). Presumably these or similar triterpenoids are
found in all depside species of Niebla.
Penicillium aurantiacobrunneum was cultured from the specimen
collected at Point Reyes (17806). Isolated from this fungal associate
were 4-epi-citreoviridin, 15 auransterol, and two analogues of
paxisterol, along with two known metabolites (Tan
et al. 2019). Additional studies continue on paxisterol; see
under Tan et al. (2019).
Among the related species that contain divaricatic acid, N.
testudinaria and N. eburnea are the
most difficult to distinguish from N. homalea. Their differences
are summarized in the following table.
|
|
N. homalea |
N. eburnea |
N. testudinaria |
|
|
|
|
|
|
Branching general |
Anisotomic. |
Anisotomic. |
Isotomic. |
|
Terminal bifurcate
branches |
Usually not evident. |
Usually not evident. |
Frequent. |
|
Branch shape lengthwise |
Sublinear. |
Variable. |
Variable. |
|
Branch shape cross-section |
Elliptic. |
Elliptic. |
Elliptic, prismatic, or
±4-angled. |
|
Branches twist |
Frequent between base and apex. |
Half twist near base and apex. |
Frequent between base and apex. |
|
Branch margin |
Usually well-defined, acute. |
Rounded to acute, usually thickened, wrinkled in upper half
of thallus. |
Variable. |
|
Cortex |
Glassy, transversely cracked at various intervals. |
Creamy frosting or like a glazed donut, irregularly
transversely cracked or pleated. |
Dull, transversely cracked and reticulate ridged
between margins. |
|
Apothecia |
Subterminal on short branch-like segments, often perpendicular to
branch margin. |
Usually subterminal, often in plane with the branch. |
Usually absent, subterminal in type, elevated from main
branch by a short flattened lobe. |
It
may also be noted that both Nylander (1870) and Howe (1913) distinguished Niebla
testudinaria from N. homalea (under Ramalina). Their studies were more
than just casual; both lichenologists monographed Ramalina. Spjut
(1996) further clarified the differences between these species by
recognizing N. eburnea, and others with divaricatic-acid and
depsidones.
The problem
in dealing with the variation in Niebla is that there are many
species in the genus that need to be sorted out before any of them can
be clearly identified. Mason Hale once asked if
there were new species at every new location I encountered Niebla. The Niebla and Vermilacinia complexes
comprise
one of the most variable and poorly understood lichen groups on the
planet. They are also vanishing; for example, a morphological variant of
N. disrupta,
distinguished in part by having sekikaic acid, was collected by Riefner
in 1986 on rocks just above the littoral around Morro Bay where he had
reportedly found it to be abundant, but I did not see any Niebla
around Morro Bay when I looked for it in Feb 2011. Additionally, conservative taxonomic views on Niebla
(Bowler & Marsh 2004)
would seem to mask many rare species of Niebla
and Vermilacinia that may need protection.
References
Acharius, E. 1803.
Methodus qua omnes detectos lichenes. Stockholm.
Acharius, E. 1810. Lichenographia
universalis. Gottingen.
Ametrano C, Y Sun, I DiStefano, S Huhndorf,
H. Thorsten Lumbsch, and F Grewe. 2021. Draft Genome of Niebla homalea. In:
Duong TA, J Aylward, CG Ametrano, B Poudel, QC Santana, PM Wilken, A
Martin, KS Arun‑Chinnappa, L de Vos, I DiStefano, F Grewe, S Huhndorf, H
Thorsten Lumbsch, JR Rakoma,, B Poudel, ET Steenkamp, Y Sun, MA. van der
Nest, MJ Wingfeld, N Yilmaz, and BD Wingfeld.
2021. IMA Genome - F15. Draft genome assembly of Fusarium pilosicola,
Meredithiella fracta, Niebla homalea, Pyrenophora teres
hybrid WAC10721, and Teratosphaeria viscida.
IMA Fungus. Open Access
Bory, St-V., de. 1828. Cryptogamie. In
Voyage autour du monde, par M. L. I. Duperrey, capitaine de Frgate.
Arthus Bertrand, Paris.
Bowler, P. A, R. E.
Riefner, Jr., P. W. Rundel, J. Marsh & T.H. Nash, III. 1994. New species
of Niebla (Ramalinaceae) from western North America. Phytologia
77: 23-37.
Bowler, P. A.
and
J. Marsh. 2004. Niebla.
Lichen Flora of the Greater Sonoran Desert 2: 368–380.
De Notaris CG, 1846. Prime linee di una nuova
disposizione de Pirenomiceti Isterini. Giornale Botanico Italiano 2,
part I, fasc. 7-8: 5-52.
Diaz-Allen, C., R. W. Spjut, A. Douglas Kinghorn, and H. L. Rakotondraibe. 2021. Prioritizing natural
product compounds using 1D-TOCSY.
Trends in Organic
Chemistry 22: 99-114.
Follmann, G. 1976. Zur
Nomenklatur der Lichenen. III. Uber Desmazieria Mont.
(Ramalinaceae) und andere kritische Verwandtschaftskreise. Philippia
3:85-89.
__________1994.
Darwin's “lichen oasis” above Iquique, Atacama Desert rediscovered.
International Lichenological Newsletter 27: 23-25.
Hale, M. E. Jr. and M.
Cole. 1988. Lichens of California. Univ. California Press.
Howe, R.H., Jr. 1913.
North American species of the genus Ramalina.
Bryologist 16:
65-74.
J Jorna, J Linde, P Searle, A Jackson,
M-E Nielsen, M Nate, N Saxton, F Grewe, M de los Angeles Herrera-Campos,
R Spjut, H Wu, B Ho, S Leavitt, T Lumbsch. Species boundaries in the
messy middle -- testing the hypothesis of micro-endemism in a recently
diverged lineage of coastal fog desert lichen fungi. Submitted March 29,
2021, to Ecology and Evolution, open access to ms,
Authorea. In this study, only Niebla species were assessed
employing RAD –sequencing.
Krog, H. & H. Østhagen.
1980. The genus Ramalina in
the Canary Islands. Norwegian J. Bot. 27:255-296.
Meyer, G. F. W. 1818.
Primitiae Florae Essequeboensis.
Gottingae: Sumptibus H.
Dieterich.
Molnára, K. and E. Farkas. 2010.
Current results on biological activities of lichen secondary
metabolites: A review. Z. Naturforsch. 65 c, 157–173.
Montagne, D.M. 1834.
Description de plusierus nouvelles espces de cryptogames dcouvertes par
M. Gaudichaud dans l'Amrique mridionale. Ann. Sci. Nat. Sr. 2, 2:369-373
& pl. 16, fig. 1.
__________. 1844.
Botanique. In Voyage de la Bonite, C. Gaudichaud & A. Bertrand, eds. Roi,
Paris.
__________. 1852.
Diagnoses phycologicae. Ann. Sci. Nat. Sr. 3, 18, 302-319.
Nylander, W. 1870.
Recognitio monographica Ramalinarum. Bull. Soc. Linn. Normandie, Sér. 2,
4:101-181.
Rakotondraibe H L R, Spjut R W, Addo E M. 2024.
Chemical Constituents Isolated from the Lichen Biome of Selected Species
Native to North America. Prog Chem Org Nat Prod. 2024;124:185-233. doi:
10.1007/978-3-031-59567-7_3. PMID: 39101985.
Rundel, P.W., P.A. Bowler & T.W. Mulroy.
1972. A fog-induced lichen community in northwestern Baja California,
with two new species of Desmazieria. The Bryologist 75: 501–508.
Rundel, P.W. and P.A. Bowler,
1978. Niebla, a new generic name for the lichen genus Desmazieria (Ramalinaceae).
Mycotaxon 6:497-499.
Sérusiaux, E., P. Van den Boom, and D. Ertz.
2010. A two-gene phylogeny shows
the lichen genus Niebla (Lecanorales) is endemic to the New World
and does not occur in Macaronesia nor in the Mediterranean basin. Fungal Biology 114:
528-37.
Sipman, H.J.M. 2011.
New and notable species of
Enterographa, Niebla, and Sclerophyton s. lat. from
coastal Chile.
Bibliotheca Lichenologica 106: 297-308.
Spjut, R. W. 1995. Vermilacinia (Ramalinaceae,
Lecanorales), a new genus of lichens. Pp. 337-351 in Flechten
Follmann; Contr. Lichen. in honor of Gerhard Follmann, F. J. A. Daniels,
M. Schulz & J. Peine, eds., Koeltz Scientific Books, Koenigstein.
_________. 1996. Niebla and Vermilacinia (Ramalinaceae)
from California and Baja California. Sida, Botanical Miscellany 14:
1–207, 11 plates.
Stevens, G. N. 1988. The lichen genus Ramalina in
Australia. Bulletin of the
British Museum (Natural History), Botany Series 16: 107–223.
Tan C.Y., Wang F., Anaya-Eugenio G.D., Gallucci J.C., Goughenour K.D.,
Rappleye C.A., Spjut R.W., Carcache de Blanco E.J., Kinghorn A.D.,
Rakotondraibe L.H. (2019) α-Pyrone and sterol constituents of
Penicillium aurantiacobrunneum, a fungal associate of the lichen
Niebla homalea. J. Nat. Prod. 82:2529-2536.
Tan CY. 2020. Identification and Dereplication of Bioactive Secondary
metabolites of Penicillium aurantiacobrunneum,
a Fungal Associate of
the
Lichen Niebla homalea. Ph.D. Dissertation, The Ohio State
University.
Anaya-Eugenio G.D., Tan C.Y., Rakotondraibe L.H., Carcache de Blanco E.J.
(2020) Tumor suppressor p53 independent apoptosis in HT-29 cells by
auransterol from Penicillium aurantiacobrunneum. Biomed.
Pharmacother.
127:110124. DOI: 10.1016/j.biopha.2020.110124.
Yamano,
Y. and L. H.
Rakotondraibe. 2022.
Understanding
the Biosynthesis of
Paxisterol in Lichen-Derived Penicillium aurantiacobrunneum
for
Production of Fluorinated Derivatives. Molecules Mar 2;27(5):1641
Aldrich L.N., J. E. Burdette, E. Carcache de Blanco,
C. C. Coss, A. S. Eustaquio,
J. R. Fuchs, A. Douglas Kinghorn, A. MacFarlane, B. K. Mize, N. H. Oberlies,
J. Orjala, C. J. Pearce, M. A. Phelps, L. H.
Rakotondraibe, Y. Ren, D. Doel
Soejarto, B. R. Stockwell, J.
C. Yalowich, and X. Zhang. 2022. Discovery of
Anticancer Agents of
Diverse Natural Origin. J. Nat. Prod. 85 (3): 702-719
DOI: 10.1021/acs.jnatprod.2c00036.
Trevisan, V. G. 1861. Ueber
Atestia eine neue Gattung der Ramalineen aus Mittel-Amerika. Flora
4:49-53.
Y.
Zhang, CY Tan, RW Spjut, JR Fuchs, AD Kinghorn, LH Rakotondraibe. 2020
(Oct). Specialized metabolites of the United States lichen Niebla
homalea and their antiproliferative activities. Phytochemistry 180,
112521. In this paper, the type locality for N. homalea is
determined to be most likely Point Reyes in Marin County, California,
collected by Archibald Menzies in November 1792. Triterpenoids in
Niebla homalea were new, and named nieblastictanes, the name given
in reference to the genus name, Niebla, and stictanes, which are
related triterpenoids, previously discovered in unrelated South American
lichens belonging to the genus Sticta.