|
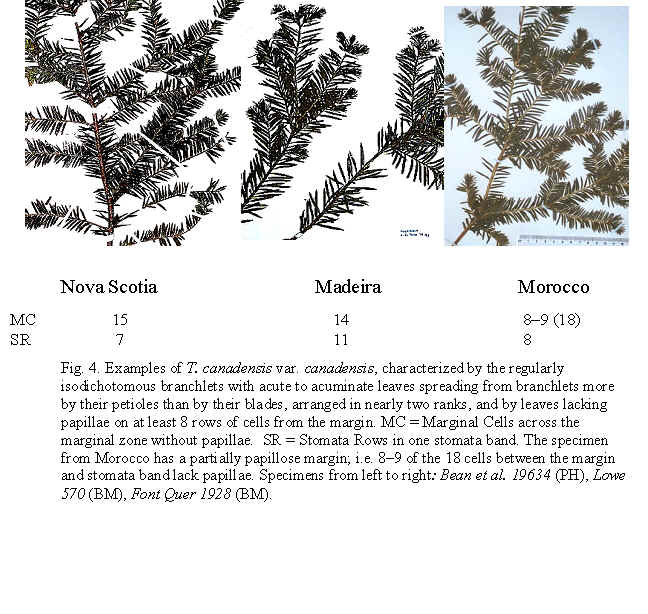
22. Taxus canadensis Marshall (Fig. 2A), , Arbust. Am. 151. 1785; T. baccata L. var. canadensis (Marshall) Gray, Man. Bot. N. United States, ed. 2, 425. 1856. Taxus baccata L. ssp. canadensis (Marshall) Pilger, Planzenreich 18 (iv, 5): 113. 1903. Original material unknown. Neotype (designated by Spjut 2007b)—U.S.A.: Vermont, Pringle, yr 1877, at US! (Fig. 275, leaf with abaxial marginal region of 16–18 smooth cells wide, stomata bands papillose throughout, stomata in 6 irregular rows/band, Fig. 276). Taxus canadensis is
distinguished by the leaves having relatively narrow stomata bands
bordered by 8 or more marginal cells.
Stomata usually number 5–7 (-9) rows/band in American plants, or
from 4–11 rows/band in Eurasian plants.
The marginal zone varies from 12–18 cells wide in America plants,
and from 8–24 cells wide in European plants.
American plants have papillae confined to stomata bands, sometimes
only along a stomata line. Except for one specimen from Ithaca NY, the Eurasian plants
also differ by more conspicuous papillae in stomata bands with papillose
cells sometimes extending into the adjacent marginal zone. The European
plants could be referred to fossil species such as T. inopinata
Givulescu or T. grandis Krausel.
The consistent lack of papillae along the abaxial marginal zone in
leaves of American plants clearly indicates that development of epidermal
papillae outside stomata bands is genetically determined Taxus canadensis has also been distinguished from other related species by its low sprawling, layering, monoecious habit, but these characters cannot be evaluated by herbarium specimens alone. Moreover, T. canadensis is not always monoecious (Allison 1991), and tree forms may be found. One European specimen was collected where the oldest trees of yew in France are thought to occur, at Sainte Baume, and where yew trees, “attaining a girth of 11˝ feet”, were reported to occur in abundance (Elwes & Henry 1906), which may be due to layering. However, layering is apparently rare in European yews, reported for yew trees of the Scandinavian region (Elwes & Henry 1906) where I also recognize T. canadensis from herbarium specimens. Two features that help confirm identification of T. canadensis outside North America are bud-scales at base of branchlets and color of leaves. In the Canada yew, bud-scales remain rather loosely attached at base of branchlets, and are usually keeled or folded along the mid nerve, often appearing incurved or cuspidate above the mid region as in T. biternata. This is in contrast to scales of the T. baccata Alliance that show various other combinations of character attributes. For example, in T. baccata var. baccata, bud-scales at base of branchlets are loosely attached, but still thick and obtuse, not at all incurved or cuspidate, whereas other varieties of T. baccata have similar thickened obtuse scales more tightly adpressed to branchlets, or in the related T. recurvata, bud-scales are thickened cuspidate but not tightly adpressed to branchlets. Only var. washingtonii appears to have bud-scales similar to T. canadensis and perhaps this is not surprising since this taxon may include hybrid plants between T. baccata and T. canadensis. The second feature that helps distinguish T. canadensis is the dark green color of leaves as seen from general observation and under the microscope. Most dried leaves of T. baccata and T. recurvata have a yellowish green, olive green, or glaucous green color except specimens from the E Mediterranean Region where color and anatomical differences overlap between T. canadensis and T. baccata var. washingtonii. A specimen of T. baccata var. washingtonii from Bosnia, for example, has yellowish green leaves that are relatively narrow with 6–8 stomata rows/band bordered by a narrow margin of mostly papillose epidermal cells (8 cells wide, 6 papillose), and seed is present on 2nd yr branchlets. Three varieties of T. canadensis are recognized. The typical variety is frequently isodichotomously branched with a leaf arrangement in dried specimens appearing crisscrossed in pairs along one side of the branchlet. The leaves are broad linear and spread from branchlets at their petioles in which the blades then remain relatively straight. Variety adpressa differs by the irregular branching and in having oblong leaves, while var. minor is recognized by the crowded erect (secund) leaves. Further studies may recognize additional varieties as may be represented by Hayek s.n. (BM) from Styria Superior in Austria, appearing distinct for its dark scales at base of branchlets, recurved branchlets, and erect leaves. It is shown below under var. minor. 22a. Var. canadensis (Figs. 138–139, 275–276). Trailing shrubs, usually without a distinct upright trunk, stems layering, leafy on the ascending parts, to 50 cm high (or upright shrubs with a much-branched woody base to 2 m high?); persistent bud scales 3-seriate, chartaceous or coriaceous, yellowish-brown, ovate to obovate, ca. 1 mm long, the lower scales plane to conduplicate along a nerved midrib, the upper-most scales slightly spreading, galeate. Leaves often dark glossy green above, paler green below, spreading and overlapping in two-rank like arrangement, or spreading somewhat in a radial manner and crisscrossing in pressed specimens, linear, straight to falcate, 1–2 cm (-2.5) cm long, 1–2 (-2.4) mm wide, relatively thin in American plants, 175–275 (-350) µm thick, convex above to a rounded to acute midrib, concave below to a rounded midrib, abruptly recurved near margins (80–90ş); with a marginal region of (10-) 12–18 cells wide in plants from North America, S Europe and N Africa, or 18–24 cells wide in plants from N and E Europe; epidermal cells nearly rectangular, 4–10 (-15)× l/w on midrib, shorter, trapezoidal, and slightly inflated near margins, or notably trapezoidal in var. adpressa, elliptical in transverse section, 10–15 µm tall, 25–40 µm wide, thin-walled, epapillose outside stomata bands in American plants, occasionally papillose in part in European plants but not more than half of the marginal cells, papillae often obscure on stomata bands in American plants, more conspicuous in European plants—except fossils; stomata bands narrower than marginal zone, with 5–6 (-7-9) rows of stomata in American plants, 4–9 (-10) in European plants, dark green. Male cone scales 3–4 seriate; microsporangia ca. 8, barely exserted from the scales at maturity, shortly spreading from a column in an umbrelliform manner; microsporangia 4 (-6), cucullate when young but spreading and becoming flat as pollen is shed, shallowly lobulate. Seed usually maturing on 2 nd yr or older branchlets, rarely on both current season branchlets and older branchlets, subcylindric to slightly obovoid, abruptly tapering near apex, 4–5 mm long. Canada yew. Distribution: shady wet places, benches above rivers, or among rocks or soil in bottomland forests of mixed hardwoods and conifers, especially hemlock and beech, 300–1500 m; E North America (Manitoba near Lake Winnipeg S to Indiana, Newfoundland S along the Appalachian Mts. to NW North Carolina and Tygarts Creek, Kentucky), NW Africa, Europe, W Asia. Representative Specimens—North America—Canada: s.loc. yr 1836 Makay (S: C-2076). Newfoundland: St. Barbe Dist., Eastern Blue Pond, Rouleau 6545 (US); Highlands of St. John, western calcareous slopes, summit, Bard Harbor Hill, Wiegland & Gilbert 27304 (PH); Prince Edward Island, Larch swamp, dundee, Fernald, Long & John 6738 (PH). Nova Scotia: Halifax Co. near Halifax, St. Margaret's Bay, near Prospect Road, Gorham 45139 (US). New Brunswick. St. John, Allen 2528 (PH). Quebec: Gasp, Co., Mt. St. Pierre, calcareous lede pockets at summit, Fernald Weatherby & Stebbins 2404 (US); Darmounth River, Tae hé, & Lepage 332 (PH); Montreal, Chas Mohr s.n. (US); Baie Missisquo, Louis-Alphonse 3547 (US); Laurentides, Bellerive, Lucien 743 (PH). Ontario: Sagastaweeki Island, McDonald 223 (US). U.S.A. —Minnesota: Towers St. Laus, Sheldon s.n. (US). New Hampshire: White Mts. Natl. For., near stateline of Maine, along road to Wildriver Campground, 300 m, Spjut 11778 (NA). Vermont: Willoughby Lake, Stevenson s.n. (US). Massacheusetts: Amherst, Herb. C. W. Minott (US). Indiana: Montgomery Co., Deam 12472 (US). Conneticut: Boston Hollow, Weatherby 5977 (US). Pennsylvania: Bucks Co., Kintersville, W. R. Taylor 424 (US). West Virginia: 900–1200 m, Allard 12060 (US). Maryland: Garrett Co., Bailing Spring, Shreeve 1971 (US); Women's College of Baltimore (US). Virginia: Palmer & King 205 (US). Kentucky: Carter Co., Cascade Caverns, shaded limestone talus, Gilbert 831 (PH).
Euro-Mediterranean—Morocco: between Mts. Kalaa and Tisuka, 1500 m, Font Quer, 15 June 1928 (BM). Portugal: Madeira, 17 Oct. 1866, ex Herb. Lowe (BM). France: Sainte Baume, ex Herb. Gombault (S: C-2043). Sweden: Södermanland, Asplund (US: long-needled specimen of 2 on sheet). Estonia: Ösel near Karriland, Lundström 742 (S); Ösel, Sworbe (Sorve), Lundström 562 (S), 579 (S). Russia—Krasnodar Region: mt. range—Markuth, near Novorossiysk, in canyon near Saharnoy golovci, N slope, 1924, Poyavcowa 496 (BH). Azerbaijan: Gadrutska Region, Nagorny Karabah, between village Dommie and pasture in oak-hornbean forest, 26 Mar 1948, Kirpichnika s.n. (BH). [Russian Federation: Caucasus]: Kuban [river], Kousnetzoff 89 (US: 254512).
22b.
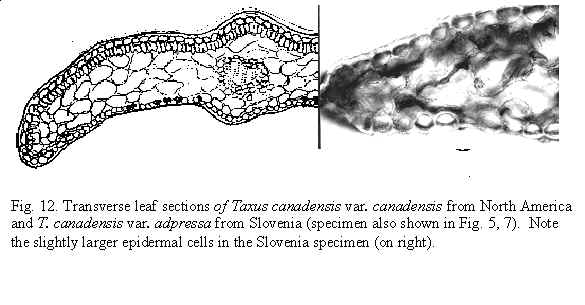
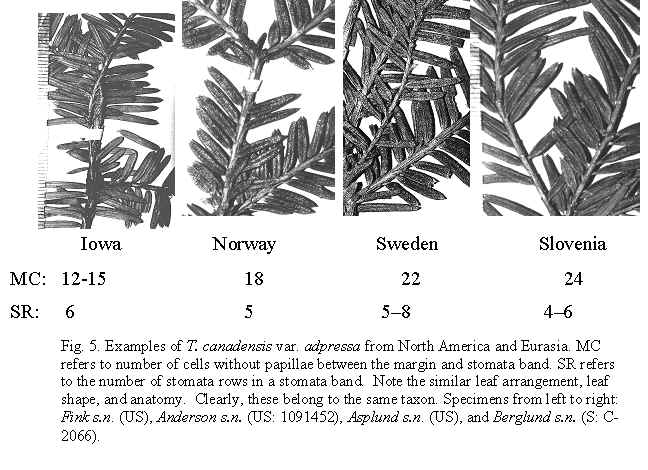
Taxus canadensis Marshall var. adpressa (Carričre) Spjut (Figs. 5, 8, 12,
140–141 277), J. Bot. Res. Inst. Texas 1(1): 279. 2007. Taxus [baccata var.] adpressa
Hort. ex Carričre, Rev. Hort., sér 4, 4: 93, fig. 8. 1855; Traité
gén. conif. 520. 1855. Taxus baccata [var.] adpressa Carričre,
Traité gén. conif. 731. 1867. Rigid-leaf yew. Distribution: E North America, Europe, W Asia. Apparently low trailing shrubs with isodichotomous branching, or erect
shrubs in apparent hybrids in cultivation (Rehder 1940). Leaves mostly in
two-ranks, thick, stiff, divaricate, oblong, obtuse, apiculate; abaxial
epidermal cells variable in shape and length, trapezoidal to rectangular,
slightly inflated in one specimen from Slovenia, the nonstomata zones
epapillose, or papillose on midrib in one specimen from England; stomata
4-8 rows/band, or to 10 rows/band in cultivars, or to 14 rows in specimens
from SE Russia. Aril not
always adhering to the seed; seed 3-anlged, often depressed at summit. The name for this taxon has been confused. If recognized as a species, the correct name would be Taxus tardiva, but as a variety, the epithet adpressa has priority. Carričre referred to it both ways in 1855, as a species in Revue Horticole and as a horticultural variety in Traité Général des Conifčres under a bionomial name (Taxus adpressa) with many synonyms including one (T. baccata adpressa) that referred to his earlier journal publication where he actually recognized it as a species. The ICBN (Art. 11.2, 11.4) indicates that priority is determined by the “final epithet,” which reference to “Taxus adpressa” as a variety even though in a binomial format still has priority over Cephalotaxus tardiva, first recognized as a species. Additionally, “Taxus adpressa” was once thought to occur naturally in California (Revue Horticole 1848, 1849), mentioned in association with sugar pine, ponderosa pine, Douglas fir and other species.
This variety is generally known in horticulture where it supposedly originated as a natural seedling in a nursery at Chester, England in 1826 (Bean 1953; Elwes & Henry 1906; Pilger 1916; Wilson 1916), and also considered occasionally wild (Krüssmann 1985), but others considered that it may have been native to China and Japan (Endlicher 1847; Koch 1873). Specimens from Japan and China that resemble this variety include an illustration for T. cuspidata in Siebold and Zuccarini (1870), a specimen I received from Phyton in Ithaca (identified by an unpublished name and reported from China), and a cultivar at the Secrest Arboretum. These are referred to T. umbraculifera var. microcarpa. Perhaps the variation in T. canadensis var. adpressa represents various derived features from a former circumboreal species (T. tardiva clade), which may include T. chinensis in South Vietnam and in Sichuan (China). An apparent autaapomorph from England (Summerhayes 2581) is similar to a specimen from South Vietnam (Chan 405) in a number of leaf character traits such as shape in surface view, shape in transverse section, in the abaxial epidermis lacking papillae across 8 marginal cells, and in the submedial position of epidermal papillae. Specimens from Mt. Emei in Sichuan (China) differ from T. chinensis in Vietnam by the leaf epidermis having conspicuous papillae along cell walls while also showing relationships to T. umbraculifera in lacking papillae across 7–12 marginal cells as seen in approximately half of the specimens (7/13); most T. chinensis and T. wallichiana lack papillae on 4 or less marginal cells across. Specimens from Norway, Sweden, and Slovenia are similar to each other and to cultivars of var. adpressa in leaf shape and anatomy, while the Slovenien specimen also differs by fewer stomata—4 (-6) rows/band and inflated epidermal cells as seen in transverse section. Taxus chinensis differs from var. adpressa in the tree habit, in leaves having 11 or more rows of stomata/band and papillae positioned marginally on epidermal cells. Perhaps the ancestral taxon was characterized as having a trailing stem with occasional branchlets and with oblong leaves truncated near apex and base.
Tardiva clade?
Taxus canadensis var. canadensis in NE America may represent another apomorph characterized by trailing stems that reproduce by layering and by leaves that have narrow stomata bands bordered by 12–24 cells without papillae. Similarly, T. umbraculifera var. microcarpa in E Asia includes flat-topped layering shrubs with leaves that lack papillae across 8–9 (-14) marginal cells while also differing in having more stomata—10–14 stomata rows/band. The leaf characters that distinguish var. canadensis and var. adpressa may intergrade as evident in specimens collected by Erik Asplund of both varieties from Sweden mounted on one herbarium sheet (US). Leaves with a papillose midrib in the European plant of var. adpressa (Summerhayes 2581) is a trait that may have been acquired through introgression with T. recurvata, whereas in Asia there may have been a former taxon with features of var. adpressa that allegedly has become modified to the extent that it is no longer distinct from T. chinensis (specimens from Mt. Emei). The specimen from Slovenia (Berglund s.n.) may have been derived from this ancestral taxon in which larger epidermal cells evolved as a result of adapting to a Mediterranean climate . Other examples where stomata have become reduced in number and the epidermal cells have become distorted in width are evident in T. brevifolia and T. mairei, allegedly derived from T. globosa, and T. celebica, respectively. Leaves of var. adpressa usually have short trapezoidal to pentagonal shaped epidermal cells on the abaxial surface, a feature that is evident in a leaf of a Pliocene fossil described by Kvaček (1984, Taxus sp. 2). This is in contrast to longer rectangular to pentagonal cells in most other yews. Rather than give taxonomic status to each variant of T. canadensis that collectively could be placed under T. tardiva, I have recognized them all under T. canadensis var. adpressa by leaf characters of shape and relative width of the stomata band as defined by the absence of papillae in the marginal region. In the Madeira Islands, in Sweden and on Saarenmaa Island in Estonia, var. adpressa appears sympatric with var. canadensis. Collectors seem to recognize this by providing specimens of both varieties as mounted on a single herbarium sheet. The Estonia records are based on herbarium specimens of var. canadensis, whereas var. adpressa is identified from a photo on a Helsinki website showing a low sprawling plant reportedly in the understory of a spruce [Picea abies] forest (Ilkka Korpela's Index, Dec. 1996, http://honeybee.helsinki.fi/users/korpela/taxus_baccata.html, and Korpela email comm. 2001, Apr 2006, site not available July 2007, photo provided below). Its low sprawling habit is much like the American Canada yew. Moreover, the only yew I have recognized in Estonia is T. canadensis. It may be further noted here that all three varieties appear to occur in the Madeira Islands. The cultivars that I observed at Kew Gardens and at the Secrest Arboretum appear to be hybrids between wild var. adpressa and T. baccata or T. cuspidata. These may represent ‘Adpressa Aurea,’ ‘Adpressa Erecta,’ ‘Adpressa Pendula,’ ‘Adpressa Pyramidalis,’ and ‘Adpressa Variegata’ (den Ouden & Boom 1965; Krüssmann 1985), which all are female plants often propagated by grafting. These cultivars are generally recognized by their thick rigid branches and relatively short truncated leaves. Representative Specimens—United States—Iowa: spreading shrub, 23 Apr 1894, fruit collected 16 Aug., Fink s.n. (US). England: Kent, yew scrub on steep slope, Summerhayes 2581 (K). Norway: Kolsaas, O. Anderson, 21 May 1907 (US: 1091452). Sweden: Asplund (US: short-needled specimen). Slovenia: Bled, berget Straza. Berglund s.n., 26 Aug. 1964 (S: C-2066).
22c. Taxus canadensis Marshall var. minor (Michx.) Spjut (Figs. 142–144, 278), J. Bot. Res. Inst. Texas 1(1): 274. 2007. Taxus baccata β minor Michaux, Fl. Bor. Amer. 2: 245. 1803. Taxus minor (Michx.) Britton, Torrey Bot. Club 4(1): 167. 1893, no specimens cited. Type: CANADA. Holotype—ex Herb. A. Michaux (Fig. 278, P!). Plants differing from var. canadensis by their apparent erect dioecious habit; shrubs with horizontal branches; leaves more radial than in two ranks, oblong to linear, acuminate, acute, or obtuse; stomata (6-) 7–9 rows/band. Minor yew. Distribution: NE U.S., E Canada, Portugal (Madeira), Austria. The type seems distinct for its relatively short (oblong) obtuse leaves, but intermediates to the typical form seem best differentiated by erect orientation of the leaves. In one specimen from Ovis Island, Maine (USA), which has wide spreading branchlets with erect leaves, the seed cones are reflexed as in T. cuspidata. Field studies are needed to the determine characteristic habit of the different varieties that is evident from herbarium specimens. A specimen at the British Museum (BM) collected by Joseph Banks in 1766 has a notation with a reference to “Marsh. Hb.” This might be interpreted as a type; however, the existence of material used by Marshall is unknown. His publication (Marshall 1785) was related to his sales catalogue (Silber & deWolf 1970). In order to make the most effective use out of the available names where types are known—and to avoid creating new names, I have designated a neotype to represent the most common variety of the species. The Banks specimen, which is shown below, is identified as T. canadensis var. minor. The wide distribution of var. minor, like var. adpressa, may date back to the Miocene or earlier as suggested by the comparative data below. Kvaček (1984) found that his Taxus sp. 1 from a Lower Miocene deposit in Bohemia compared most favorably with a modern specimen of Taxus canadensis from Maine. Spjut (2007b) interpreted both of these as belonging to var. minor, which also includes specimens from Austria and from the Madeira Islands. As shown in Spjut (2007a, 2007b), the similarities go beyond the leaf arrangement and shape; they are also character attributes of leaf anatomy. Based on data in Spjut's (2007) phytogeographical analysis of Taxus, the chances that any two specimens selected at random from the Euro-Mediterranean region will be found to share both gross morphological and leaf anatomical features as recognized for var. minor are less than 1 in 10,000. Although molecular studies have yet to confirm these conclusions, it seems reasonable to conclude that Taxus canadensis and its varieties have changed little since the Neogene. Moreover, assuming that these findings are supported by molecular data, a molecular taxonomists would have to analyze more than one million specimens at random to be able to show the same results for three specimens. As indicated in Spjut (2007b), molecular studies of Taxus have been mostly geographically based because the investigators have assumed that morphological character attributes of Taxus are too variable (Kadkade pers. comm.; Krupkin pers. comm.). Representative Specimens—U.S.A.—New York: Coy Glen, Ithaca, Eames 3432 (US). Conneticut: Meriden Hills, Safford 308 (US). Maine: Rockport, Fellows 5686 (US); Casco Bay, Ovis Island, head of Long Cone, True 164 (PH). Canada—Quebec: Montreal, Chas Mohr s.n. (US). Nova Scotia: Victoria Co., Timber Brook (S: C-2156). Newfoundland: Bay Islands, Palmer 1300 (US); Buochan, Jaussan 292 (S: C-2130); Banks, collected 1766 (BM). Portugal: Madeira, Dec. 1895, ex Herb. Lowe ex Barby (US).
|





































 Cephalotaxus tardiva Siebold ex
Endlicher, Syn. conif., 239. 1847. Taxus tardiva (Siebold ex
Endl.) Hort. ex Knight, Syn. conif. pl. 52 (1850); T. baccata
f. tardiva Pilger, Pflanzenreich 18 (iv, 5): 114. 1903.
Cephalotaxus tardiva Siebold ex
Endlicher, Syn. conif., 239. 1847. Taxus tardiva (Siebold ex
Endl.) Hort. ex Knight, Syn. conif. pl. 52 (1850); T. baccata
f. tardiva Pilger, Pflanzenreich 18 (iv, 5): 114. 1903.