|
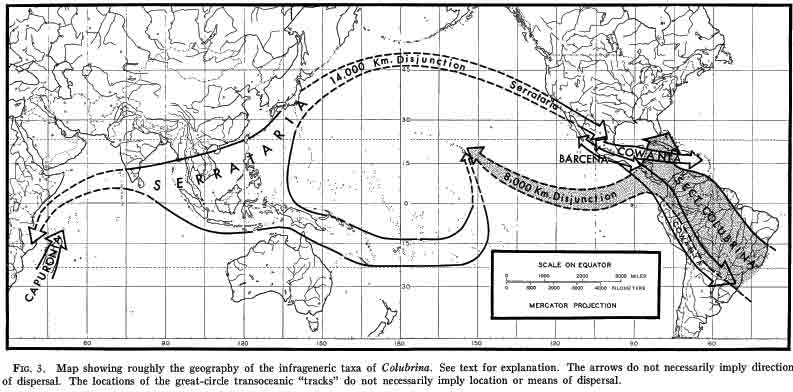
Worldwide distribution of Colubrina, M. C. Johnston, 1971. Revision of Colubrina (Rhamnaceae). Brittonia 23: 1–53 (31 species). Johnston (1971) distinguished 31 species in the genus Colubrina. Since then, Wendt (1983) described C. johnstonii from southern Mexico; Van Wyk and Schrire (1986) discovered a rare species, Colubrina nicholsonii, endemic to South Africa; Palacios (2015) recognized C. amazonica from the Amazon of Ecuador; Nesom (2013) elevated two of Johnston's (1971) varieties of the North American C. greggii to species; Yilin Chen and Carsten Schirarend (2007, in Flora China) re-established C. javanica Miquel 1856 formerly included under a widely distributed, back-mangrove species, C. asiatica (Ceanothus asiaticus Linnaeus 1751) Brongniart 1826 (Johnston 1971), and Figueiredo (1995) transferred the Madagascan-Comoros Lasiodiscus articulatus Capuron 1966 to Colubrina. Additionally, Colubrina anomala King 1896 was recently proposed to be synonymous with C. becarriana Warburg 1891 (Slik 2009); however, C. anomala will be recognized in this review—of the phytogeography of the genus. Although Johnston (1971) distinguished C. anomala from C. beccariana by proportionately longer leaves—4.0 or more times longer than wide (8.5-20.3 cm long x 22-52 mm broad), compared to 3.6 times longer than wide for C. beccariana (12-22 cm long x 40–85 mm wide), other differences are evident in his descriptions; for example, C. beccariana leaf margins are slightly revolute, and the minute teeth are black-glandular at the tip.
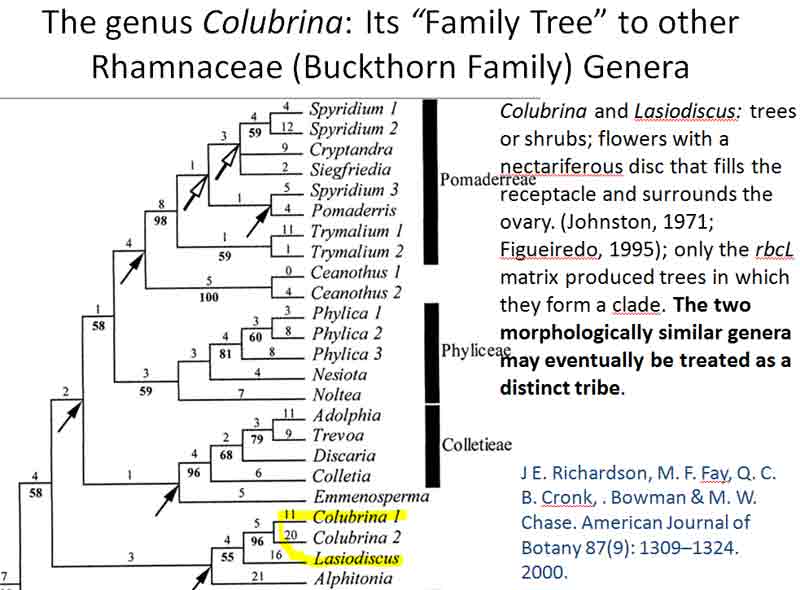
The genus Colubrina was regarded by Johnston (1971) to have
most of the ancestral character features of the family Rhamnaceae. A molecular phylogenetic
analysis by Richardson
et al. (2000) shows
Colubrina basal in a clade sister to Lasiodiscus, within a
larger complex group of clades that includes Elaeagnaceae nested within
the Rhamnaceae
based on the rbcL
[RuBisCO large subunit]
chloroplast gene;
the clade that included Colubrina
is shown below. They further suggested that Lasiodiscus and Colubrina might be classified in a separate tribe.
Lasiodiscus, largely an African genus of approximately 13
species (Figueiredo 1995), is distinguished by the seed lacking
endosperm, and by the stems usually having interpetiolar stipules
(Johnston 1971). |
|
|
|
|
|
|
|
|
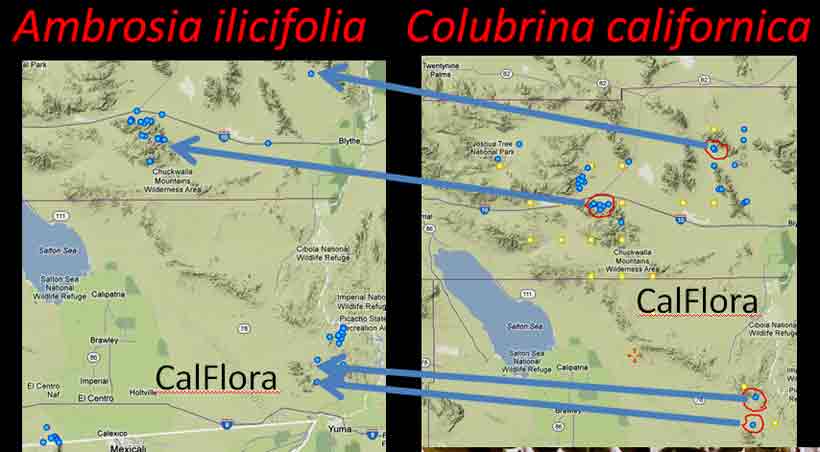
Figueiredo (1996) concluded from a cladistic analysis of morphological characters that Johnston's (1971) infrageneric classification of Colubrina could not be upheld. This included the presence of an aril, a key character that Johnston used to define four species endemic to Madagascar (Section Capuronia), except that the aril is not always evident in Sect. Capuronia (Figueiredo 1996). Other morphological characters employed in the cladistic analysis of five endemic Madagascan species—that included C. articulata—did not group into a clade. This raises the question as to what distinguishes the five Madagascan species of Colubrina from the rest of the world species, for example, Colubrina humbertii in Madagascar and Colubrina californica in the Sonoran Desert? Do each of the Madagascan species of Colubrina have independent relationships to species outside Madagascar? Whether the Colubrina clade belongs to one genus, or two genera, or possibly three genera, its widely disconnected distribution is remarkable. The question of whether the species of Colubrina on different continents and islands had dispersed over long distances via air or water as opposed to gradually spreading over-land followed by vicariance is addressed below from their ecogeographic relationships to genera in other families. The focus will be on the legume subfamily Detarioideae, formerly in Caesalpinioideae, a rather heterogeneous subfamily that has since be revised by the LPWG (in Taxon, Feb 2017). The relationships of the genera will also be referred to as an association in a narrow synecological sense since the same genera occur together at disjunct locations represented by different species. Plant associations therefore are generally defined here simply as species, genera or families that occur together or in the same assemblage of plant communities more often than not. They do not necessarily conform to quantitatively defined vegetation types, but statistically could be defined and classified from an ecogeographic perspective. An example is the association between Colubrina californica (multi-stemmed shrub 2–5 m, CNPS rarity rating 2.3, or tree with single bole to 10 m, 8 mi ne of El Arco Baja Calif Norte) and Ambrosia ilicifolia (low multi-stemmed shrub, not listed by CNPS), species that apparently are too rare or too uncommon to be mentioned in A Manual of California Vegetation (Sawyer, Keeler Wolf and Evens, 2nd ed., 2008).
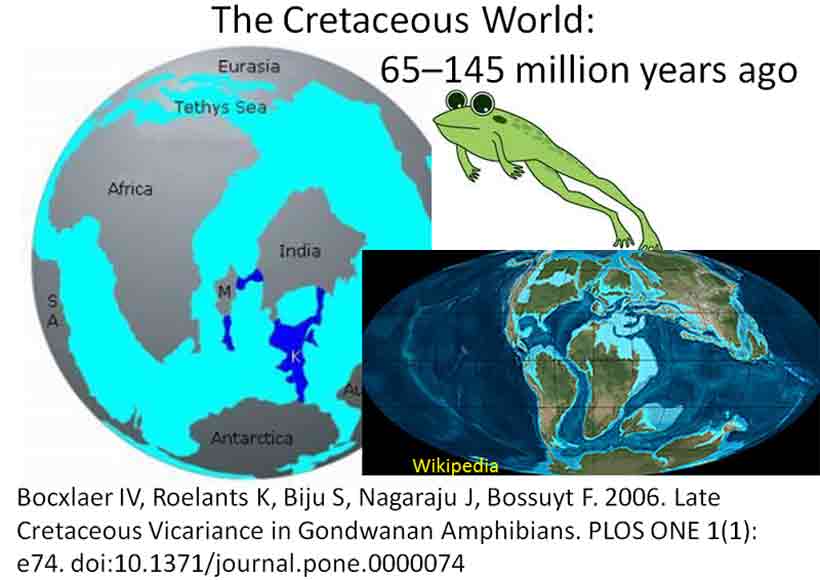
Looking at Calflora distribution maps for Ambrosia ilicifolia and Colubrina californica, they appear closely associated in their occurrences at four of five general locations in southern California (Piacho Mts., Chuckwalla Mts., Eagle Mts., Little Santa Maria Mts., A. ilicifolia only at Signal Mt.) and appear to occur together at four specific sites, but not necessarily in the same vegetation alliance. For example, in a north-facing canyon of the Chuckwalla Mountains south of Desert Center, Ambrosia ilicifolia occurs abundantly along the bottom of the rocky wash, whereas Colubrina californica occurs frequently above the wash along the base of steep rocky slopes (pers. obs.). The Ambrosia might be included in the Hyptis emoryi Shrubland Alliance, and the Colubrina in the provisional Simmondsia chinensis Shrubland Alliance (Sawyer et al. 2008). In consideration to their rarity and geographical occurrence in close proximity, they are regarded here as an example of a rare plant association. Phytogeographers commonly invoke "long distance dispersal" as the explanation for disjunct occurrences of genera across wide oceanic barriers (Raven & Axelrod 1974; Richardson et al. 2004), because it seems that the extant genera must have evolved after the break-up of Gondwana; however, the timeline for the break-up, the configuration, and the orientation of the ancient land masses also seem debatable (Coffin & Rabinowitz 1987; Noonan & Chippindale 2006). Indeed, the fire adapted (serotinous) species in the floras of Western Australia and South Africa, generally viewed as having independently evolved since the Paleogene, are also suggested to have originated during the Cretaceous on Gondwana (Lamont & He 2012); see also introduction to antitumor screening of Western Australian plants in Spjut (2014). Disjunct continental distributions have been especially problematic for fresh water amphibians because they cannot just swim in the salty ocean; they either have to take one giant leap for frog-kind, or land bridges had to have existed for them to cross ocean barriers, or their distribution patterns relate to ecogeographic differences before the continents separated. Land bridges are what Bocxlaer et al. (2006) proposed. Some frogs who did not make the leap and stayed behind got fossilized, such as Beelzebufo, a 70 million year old giant frog who has living relatives in South America.
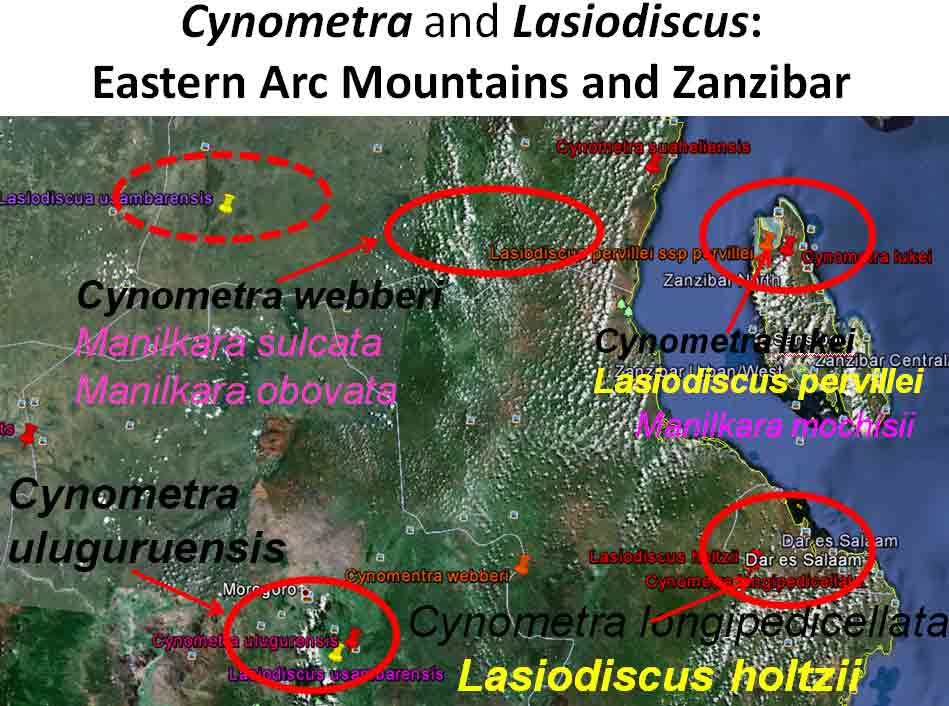
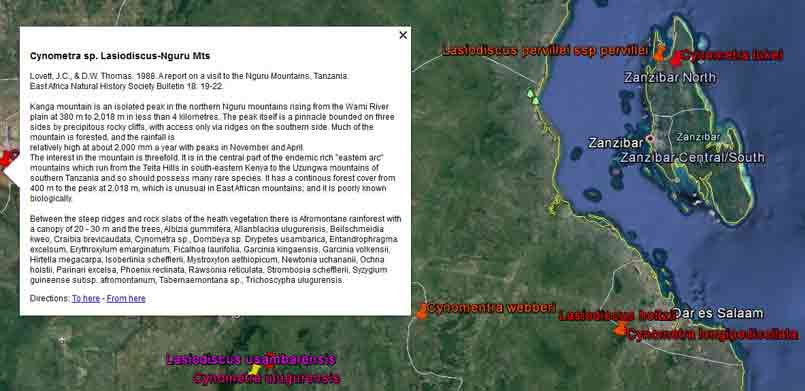
In the case of Colubrina humbertii and C. californica that grow in semi-desert environments, it would have to be aerial dispersal, either by wind or by migratory birds, but neither seem likely. The 3-carpelled fruits of Colubrina are generally coccaria (Spjut 1994); the carpels first separate from one another before opening along their dorsal sutures, and may also open halfway along ventral sutures in some species (Brizicky 1964; Johnston 1971, Fig. 1, “Colubrinoid dehiscence”). Fruits often persist on a plant 3–6 months before dehiscing, in contrast to shorter maturation of coccaria with explosive dehiscence in the related genus Ceanothus. Migratory birds might be suspected to feed on the monocarps (“endocarpids”, Brizicky 1964), each of which contains a single small seed; however, migrant birds are generally associated with wetlands (Les et al. 2003; Wikipedia). Examples of African and American desert disjunctions in genera belonging to other plant families—thought to be the result of long distance dispersa (Renner 2004)—are Thamnosma (Rutaceae) and Menodora (Oleaceae). However, in view of the Colubrinoid ecogeographic distribution patterns to be presented, it also seems likely their disjunctions could be the result of dispersal over land followed by extinction. One species of Colubrina is clearly adapted to seed dispersal by water, Colubrina asiatica, a coastal tropical species found in a “back-mangrove” plant community whose associated species are also widely distributed. The C. asiatica seeds are impervious to salt water and contain air space, allowing them to float in the ocean for many months, thus, easily transported to other lands by ocean currents (McCormick 2007, review; Johnston 1971). But the taxonomy of the genus does not indicate that all Colubrina species descended from C. asiatica; the species is unlike most in the genus in its scandent habit. Seeds of C. oppositifolia, a species that is endemic to the Hawaiian Islands and related to the widely distributed C. glandulosa in tropical America (Johnston 1971), lack air space and do not float in salt water (McCormick 2007, review). Colubrina and Lasiodiscus collectively include about 48 species. The related genus Ceanothus, by comparison, has more species in North America, ~55 spp., 46 of which are in California (Wilken 2012). Although plant geographers often invoke long distance dispersal to explain disjunct occurrences of plant taxa, it would be difficult to accept plant communities being collectively dispersed over long distances; therefore, the only logical explanation is dispersal over land followed by vicariance, which may date from the time of Gondwana. Madagascar seems to be at the center of diversity for a Cynometra (Detarioideae)-Colubrina (including Lasiodiscus) association; however, Radosavljevic (2019) proposes to exclude African species, and suggests that Madagascar is a secondary center to the primary center in the Indo-Pacific Region. Lasiodiscus-Detarioideae Associations in General As redwood, Douglas fir, ponderosa pine, and other conifers form monospecific vegetation Alliances in North America (Sawyer at al. 2008), single dominant species frequently occur in tropical African vegetation types (Richards 1952). Legume trees are often major dominants of old growth African tropical forests, particularly Detarioideae, occasionally Caesalpinioideae (Richards 1952, as Mimosoideae). Most if not all species of Lasiodiscus and Colubrina in tropical regions occur as understory trees or shrubs in Casesalpinoideae forest types, but not at all locations. Cynometra megalophylla is an example of a Detarioideae species that forms single-dominant forests in west tropical Africa where at one site in Ghana, the understory included Manilkara obovata (Sapotaceae), Alstonia boonei (Apocynaceae), Hymenostegia afzelii (Detarioideae), Memecylon (Melastomataceae), Salacia (Celastraceae) and others, but not Lasiodiscus (Enti & Spjut 1976, herbarium specimens & labels). However, Lasiodiscus does occur in the understory of Cynometra-Manilkara forests in central and eastern Africa as detailed below. In southeastern Cameroon, Lasiodiscus mannii occurs in the understory of Detarioideae Scorodophloeus zenkeri, Afzelia bipendensis, and Dialioideae Dialium, and in some transects, L. mannii was a major understory tree (Yasuoka 2009), but in other areas of Cameroon, L. mannii occurs in lowland humid forests less represented by Detarioid species (Letouzey 1968), often characterized by the presence of Annonaceae (Greenwayodendron as Polyalthia suaveolens), Cannabaceae (Celtis spp., notably C. mildbraedii), Ebenaceae (Diospyros spp.), Irvingiaceae (Irvingia spp., Klainedoxa gabonensis), Meliaceae (Entandrophragma spp. notably E. cylindricum, Khaya spp., Lovoa trichilioides), Sapotaceae (Baillonella toxisperma, Manilkara spp.), and Violaceae (Rinorea spp.). In Gabon, Lasiodiscus mannii and L. marmoratus occur frequently in permanent to seasonally inundated forests on flood plains or along rivers along with Detarioid Baikaea, Berlinia, and Gilbertiodendron. Cynometra mannii, Crudia klainei, and Manilkara obovata (Sapotaceae), Phoenix reclinata (Arecaceae) and Oxystigma mannii (Detarioideae) occur on the fringe of tidal swamps in transitions to non-tidal swamp forest (Anonymous). This may be compared to Cynometra iripa or C. ramiflora back mangrove association with Colubrina asiatica in southeastern Asia; C. iripa distinguished from C. ramiflora by the irregular mammillary fruit projections (Meeuwen 1970, Fig. 1C), both widely distributed. Lasiodiscus gillardinii was a major understory tree of the single dominant Michelsonia microphylla (Detarioideae) forests in the eastern Democratic Republic of Congo (CJB-African Plant Database). The Michelsonia (monotypic) forests reportedly have largely been destroyed by logging; thus, an entire genus and whole ecosystems are being wiped out of existence by Homo sapiens. Lasiodiscus fasciculiflorus was noted to be an understory tree of the Cynometra-Staudtia (Myristicaceae) forest in the Congo Region (Leonard 3816, K). In Cameroon Lasiodiscus mannii also occurred in the understory of Staudtia among Cola lateritia (Sterculiaceae), Combretodendron macrocarpum, Greenwayodendron (Polyalthia) suaveolens, Rinorea sp., and others (Letouzey 1968). Lasiodiscus mildbraedii was a major understory tree of the Podocarpus-Baikiaea (Detarioideae) forest in Uganda (Lind & Morrison 1974; Philip 424, Dec 1950, K) and in Tanzania (William 40, 17 Oct 1953, K. only Podocarpus mentioned). As noted later a similar type of Podocarpaceae-Myristicaceae association occurs in southern India where rare species of Cynometra and Colubrina occur in the Western Ghats. Lasiodiscus associations with Cynometra and Manilkara 1. Lasiodiscus ferrugineus and L. mildbraedii with Cynometra in Central and East African lowland rain forests. Eggeling (1947) indicated that Cynometra alexandri in the Budongo Forest of Uganda becomes dominant over a period of possibly several hundred years to form a “climax forest” of almost pure stands. Lasiodiscus mildbraedii was shown as a common understory tree, occurring with Celtis spp. (Cannabaceae) spp., Rinorea ardisiaeflora (Violaceae) and others. Eggeling (1951), in his Indigenous trees of the Uganda Protectorate, noted that Lasiodiscus mildbraedii was most common in the Cynometra alexandri forest. This close association was also noted by Evrard (1960) in the eastern Congo. Cynometra alexandri may gradually become dominant through allelopathy and mycorrhizal associations, especially ectomycorrhizae (McGuire 2007) on infertile soils with legume genera in the Detarieae-Amherstieae tribes (Polhill et al. 1981). The fungi associations involve basidiomycete families Cantharellaceae, Boletaceae, Russulaceae, Amanitaceae, and Sclerodermataceae (Onguene 2000), while arbuscular mycorrhizae were also suggested for Cynometra (Onguene 2000). Perhaps species of Lasiodiscus are more tolerant of toxic secondary metabolites in the soil produced by Detarioideae, or perhaps the soil becomes depleted of nutrients, particularly phosphorous (Moomaw 1960) leading gradually to fewer understory species in which those such as Lasiodiscus best survive. Lasiodiscus ferrugineus, considered by some to be the same as L. mildbraedii (e.g., Faden 1976) or a subspecies of L. pervillei (Figueiredo 1995), is relatively rare, occurring at scattered locations in coastal forests of eastern Africa. It has been collected in the understory of a nearly pure Cynometra webberi forest (Dale & Greenway 1961) just west of Malindi, Kenya (Spjut 3940, herbarium specimen, 1973), and as an understory shrub of a Rinorea forest with Manilkara (Greenway & Rawlins 8954, K) near a Cynometra forest in the Lamu District (Kenya National Environment Secretariat 1985), separated from Malindi by ~ 70 air miles. The intervening area between these forests and the nearest Cynometra forest to the west, the Budongo Forest in Uganda—separated by ~625 air miles—is occupied largely by savanna, thicket and woodland. Rinorea was also a major constituent of the Budongo Cynometra forest and in a Cameroon relevé ~50 km west of Deng Deng, but with Lasiodiscus mannii (Letouzey 1968). The Cameroon and Congo forests share Celtis mildbradii and species of Manilkara as also the Malindi (Kenya) and Ghana forests. Unlike many Cynometra species, the fruits (legumes) of the disjunct Congo-Kenya Cyn. alexandri-Cyn. webberi are dehiscent. 2. Cynometra and Lasiodiscus rare endemic associations in the Eastern Arc Mountains of Tanzania. Data for the map below was complied from literature and herbarium specimens. The Cynometra webberi-Manilkara sulcata forest shown in Tanzania also occurs in Kenya as noted above (Burgess et al. 1992; Shaka & Msangi 1996). Manilkara obovata was also a major understory tree in the Ghana C. megalophylla forest. Aside from this, Cynometra uluguruensis is only known from the Uluguru Mountains and while Lasiodiscus usambarensis also occurs within the same general area, it is apparently not specifically found with C. uluguruensis, but they do occur at the same elevation. Cynometra longipedicellata and Lasiodiscus holtzii are endemic to the Pugu Hills, an area generally dominated by forest of the Caesalpinioid Scorodophloeus fischeri, Antiaris toxicaria (Moraceae), Malacantha alnifolia (Sapotaceae), and Manilkara sulcata or the Caesalpinioid Dialium holtzii (Burgess et al. 1992).
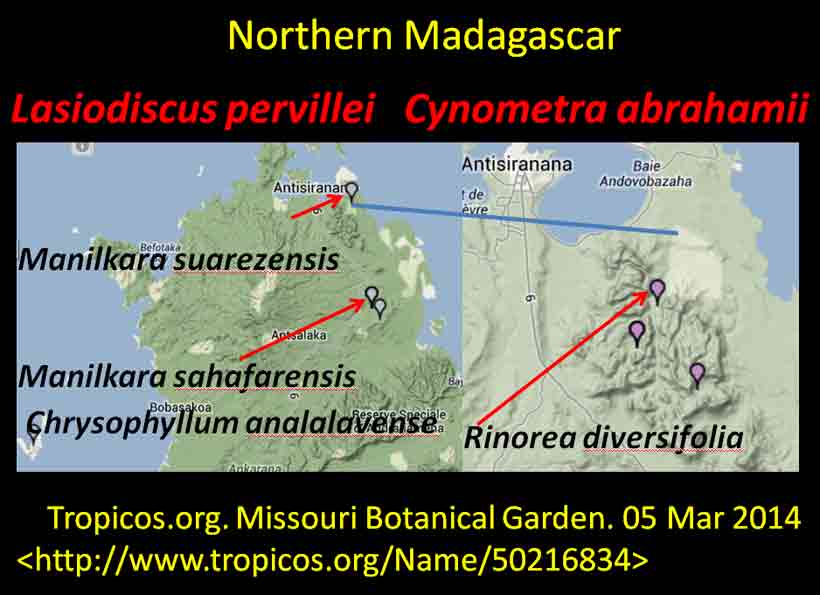
Cynometra–Lasiodiscus associations plotted on Google Earth. Four shown here for Tanzania: Upper to lower right—(1) northern Zanzibar, C. lukei and L. pervillei ssp. pervillei; (2)—Pugu Hills, C. longipedicellata and L. holtzii; lower to upper left—(3) C. uluguruensis and L. usambarensis, Uluguru Mts.; (4)—Cynometra and Lasiodiscus spp. indet., Nguru Mts. References were noted under Google Earth pin properties, occasionally with details on vegetation as exemplified for Nguru Mts., area #4. Most species endemic to pin sites; exceptions are L. usambarensis and L. pervillei; the later has another subspecies in northern Madagascar. 3. Lasiodiscus pervillei (MBG distribution data; Johnston in Flora of Tropical East Africa, 1972) and Cynometra lukei in northern Zanzibar. Both Lasiodiscus and Cynometra occur in the Inhambane Forest in northern Zanzibar, an area with a long history of human disturbance from agriculture and burning, perhaps as long as 100,000 yrs (McGinley–World Wildlife Fund 2014). This area is has been recognized as the Zanzibar-Inhambane Bioprovince, which is referred to the Northern Zanzibar-Ihambane coastal forest mosaic . What's left of the native vegetation is patches of forest that may be dominated by any one or more of the following legume species: Afzelia quanzensis, Scorodophloeus fischeri, Dialium holtzii, Hymenaea verrucosa, Millettia stuhlmannii, Berlinia orientalis, Cynometra lukei, and Xylia africana. Other sources have reported Lasiodiscus pervillei was common in the Inhambane forest (e.g., Greenway 1206, 28 Jan 1929, K), also identified by others as L. usambarensis. Recall from above that L. mannii occurred in the understory of Scorodophloeus zenkeri, Afzelia bipendensis, and Dialium in southeastern Cameroon. Madagascar Madagascar has more Cynometra, Colubrina and Lasiodiscus species concentrated into a smaller geographical area than in other regions of the world, and they all appear relatively rare at the specific sites where they are found; i.e., they apparently have not been reported to form constituents of the vegetation types, judging from not being mentioned in Keochlin et al. (1974). Their associations thus appear more of a geographical nature rather than of a plant community type, comparable to the Colubrina-Ambrosia example mentioned above. However, Cynometra spp. may have formed a major component of the Madagascar vegetation in the past, such as from 2,410 to 910 yrs ago (Virah-Sawmy et al. 2009). In northern Madagascar, Lasiodiscus pervillei occurs near Cynometra abrahamii as indicated by the blue line shown below on maps (not proportionate in size). The mapped occurrences are generated by the Missouri Botanical Garden website for Madagascar based on their specimen locality database. They were generated individually for each species that were combined into single images for this presentation. One of the three localities for C. abrahamii has Rinorea diversifolia. Other species in Rinorea were noted above to occur as understory trees in the Cynometra forest in the Kenya Lamu Forest, in the Uganda Budongo Forest, and in several Cameroon vegetation transects (Letouzey 1968).
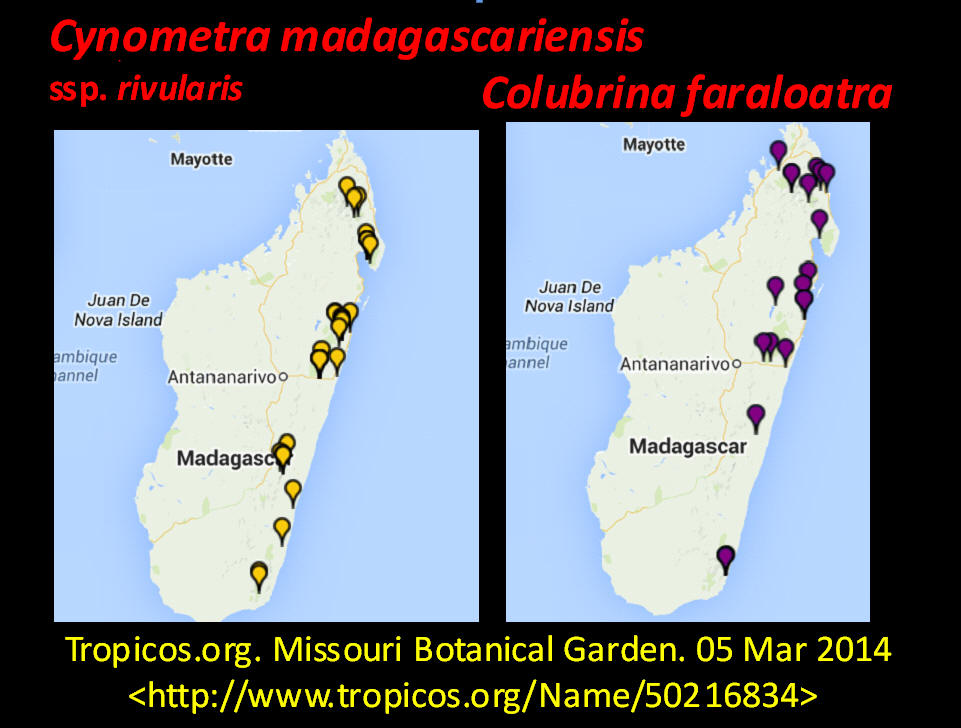
Species of Manilkara that occur at two sites with Lasiodiscus pervillei in Madagascar are also found with Cynometra in Tanzania and Kenya. Another associate species shown on the Madagascar map, Chrysophyllum analalavense (Sapotaceae), is found with Lasiodiscus pervillei, which also occurs on Zanzibar. Related species in these genera (Cynometra, Chrysophyllum, Rinorea, Manilkara, Lasiodiscus) were noted in the Kenya Lamu Forest (MBG data, 05 Mar 2014; Greenway & Rawlins 8954, K). In the Tolagnaro region of southeastern Madagascar, Colubrina faraloatra var. glabrescens was present with Cynometra cloiselii, Cyn. commersoniana, and Cyn. dauphinensis in the Madena Forest, characterized by Rabenantoandro et al. (2008) as having frequent Intsia bijuga (Detarioideae), Homalium albiflorum (Flacourtiaceae), Uapaca louvelii (Euphorbiaceae), U. littoralis, Eugenia cloiselii (Myrtaceae), E. emirnensis with emergents Canarium bullatum ined. (Burseraceae), Trilepisium madagascariensis (Moraceae), Uapaca littoralis (Euphorbiaceae), and Macphersonia radlkoferi (Sapindaceae). In southeastern Cameroon, Lasiodiscus marmoratus was found with Uapaca guineensis, U. paludosa and other Euphorbiaceae trees, Detarioideae Berlinia confusa, Dacryodes instead of Canarium, and Annonaceae trees, but not with Cynometra (Letouzey 1968). Among the ~ 10 Cynometra and five endemic Colubrina species in Madagascar, Cynometra madagascariensis and Colubrina faraloatra compare most closely in geographical occurrences of the species within the two genera.
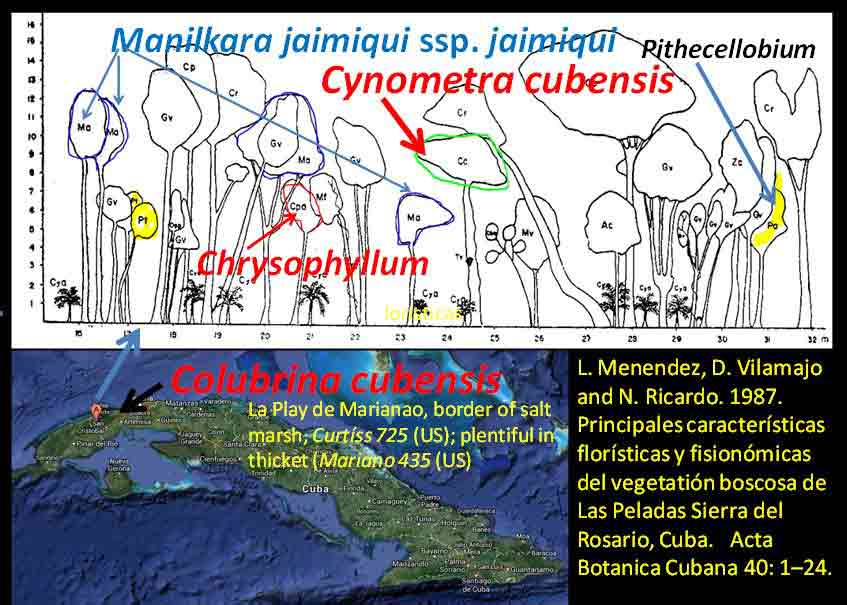
Asia and Pacific The occurrence of Colubrina with Cynometra in Asia is relatively rare except for one common and widespread back-mangrove association, Cynometra iripa and Colubrina asiatica (Kurz 1875; Stone 1970); the latter also occurs in Madagascar. Both species have fruits or seeds adapted to buoyancy for long distance dispersal by water. Colubrina asiatica has invaded Florida and Hawaii after introduction into the Caribbean region around 1850. India. In southern India, Cynometra travancorica and Colubrina travancorica are rare and endangered species that have been reportedly collected from one area in common along the Western Ghats. This region includes “primeval” Myristicaceae swamp forests of Myristica spp. and Gymnacranthera canarica, which appear to be remnants of “unique evergreen forest patches” that include Nageia wallichiana (Podocarpaceae) “facies” (Chandran et al. 2008). In Uganda and Tanzania Lasiodiscus is found in Podocarpus swamp forests with the Caesalpinioid Baikaiea, or in central Africa Lasiodiscus occurs as an understory tree to the Myristicaceae Staudtia forest as noted above. Champion and Seth (1968) recognized “Myristica swamp forest” as “restricted to valleys in the tropical evergreen forest of Travancore, Kerala.” Genera common in the African Cynometra-Lasiodiscus forests that are mentioned in Western Ghats forests within the Kerala region include Calophyllum, Chrysophyllum, Dialium, Diospyros, Drypetes, Mitragyna, Polyalthia, Pterocarpus, Sterculia, Terminalia, and Xylia. A rare Detarioid Kingiodendron pinnatum, endemic to the Western Ghats, is also present. Colubrina travancorica has been reportedly discovered (Anonymous) in the Parambikulam Tiger Reserve along with another species, one of which is in a monotypic genus thought to have been extinct, Haplothismia exannulata (Burmanniaceae). It may be also noted that an unusual endangered "purple frog" that occurs in the Kerala region has its closest relatives in the Seychelles (Sooglossidae family), a lineage that dates back 182 Mya. Malay Peninsula. Colubrina anomala (included under C. beccariana by Slik 2009) is evidently rare, reported from two locations on the Malay Peninsula. Wyatt-Smith (1964) in a general outline on the vegetation of Malaya recognized a Caesalpinioid forest deficient in dipterocarps, Cynometra-Intsia with frequent Sindora, occurring in the foothills of the Main Range. However, an association of the Malayan Colubrina anomala with Cynometra is questionable since Colubrina anomala was described as a tree occurring in mixed dipterocarp forests along rivers and on hillsides up to 100 m elevation (Slik 2009-onwards); however, it may be more closely associated with Cynometra at the southern occurrence on the peninsula. It may also be noted that in southern Madagascar Lasiodiscus pervillei occurs with the paleotropical Intsia bijuga, and that the genus Sindora, largely an Asian genus of 12 species, includes one species that occurs in Gabon. Christmas Island. Colubrina pedunculata is endemic to Christmas Island. Cynometra ramiflora, which has spongy flotation fruits similar to C. iripa, occurs on the island but not with Colubrina pedunculata (Atlas of Living Australia website). Hawaiian Islands. Colubrina oppositifolia, endemic to the islands, differs in having opposite leaves. Cynometra does not occur in Hawaii. Colubrina oppositifolia can be codominant with Diospyros sandwicensis (Ebenaceae) in an open canopy forest on ridges, which may include Caesalpinia kavaiensis and Erythrina sandwicensis (Faboideae) among a rich assemblage of woody species, or just present in a "Erythrina Forest" or in a diverse forest type with no clear dominant species in which the composition is deficient in Fabaceae (Wagner et al. 1999). Cuba, Puerto Rico, Costa Rica Cuba: Colubrina cubensis and Cynometra cubensis are found with species of Manilkara and Chrysophyllum in Cuba as also in Africa and Madagascar as already mentioned above. Colubrina cubensis, which is reported on herbarium specimen labels (US Natl. Herb.) to occur in the vicinity of Cynometra cubensis, was not mentioned by Menedez et al. (1987) to occur in the Las Peladas Sierra del Rosario forest. Pithecellobium (Caesalpinioideae) is also found with Colubrina spp. in thorn forests of Venezuela, Sinaloa and central Baja California as noted later.
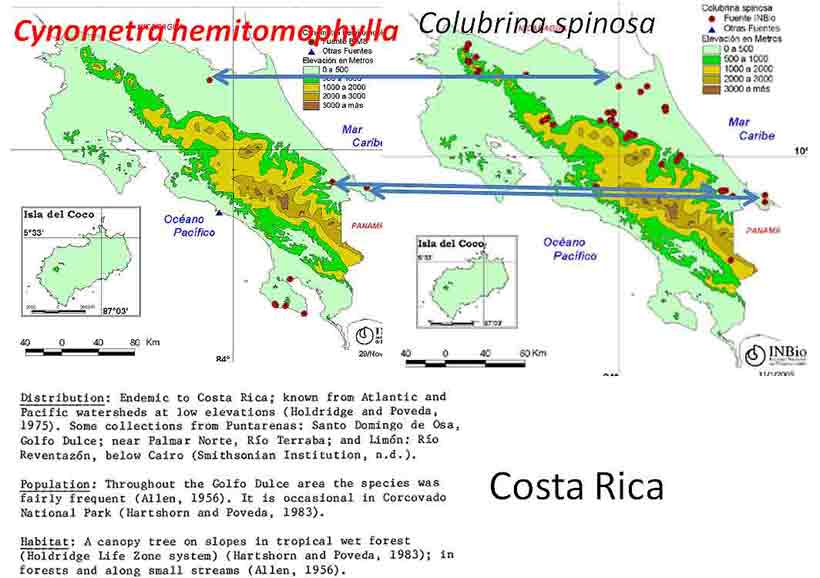
Puerto Rico: Cynometra portoricensis, a rare species in Puerto Rico and Dominican Republic (Dwyer 1958), occurs with Colubrina arborescens, a widely distributed Caribbean species. The two species are found in the same general area on limestone in the north-central part of Puerto Rico. Costa Rica: Cynometra hemitomophylla and Colubrina spinosa appear to occur at two locations and nearby at a third location in rain forests without a distinct dry season (INBio).
Cynometra hemitomophylla is noted to be canopy tree of frequent occurrence (INBio) with Esenbeckia pentaphylla (Rutaceae), Forchhammeria trifoliata (Capparaceae), Manilkara spectabilis, and others (Hammel et al. 2003-). Colubrina spinosa occurs in forests with variety of arborescent palms among a diverse canopy dominated by Ampelocera macrocarpa (Ulmaceae), Aspidosperma spruceanum (Apocynaceae), Balizia elegans (Mimosoideae), Carapa guianensis* (Meliaceae), Ceiba pentandra* (Malvaceae), Chrysophyllum columbianum*, Hieronyma alchorneoides (Phyllanthaceae), Hymenolobium mesoamericanum (Faboideae), Lecythis ampla (Lecythidaceae), Otoba novagranatensis (Myristicaceae), Pentaclethra macroloba* (Caesalpinioideae), Pouteria calistophylla (Sapotaceae), P. torta, Qualea paraensis (Vochisiaceae), Sacoglottis trichoghyna* (Humiriaceae), Sclerolobium costariense (Tachigali Caesalpinioideae), Stryphnodendron macrostachyum (Caesalpinioideae), Terminalia oblonga* (Combretaceae), Vatairea lundellii (Faboidae), Virola koschnyi (Myristicaceae), V. sebifera, Vochysia allenii and Vo. ferruginea (Hammel et al. 2003-). The lower stratum includes species of Rinorea* among others. Those denoted by an asterisk have related species in Cameroon (Letouzey 1968), but rarely occur with Lasiodiscus or Cynometra. Notable exceptions are Sacoglottis with Cynometra hankei, and Rinorea and Terminalia found with both Cynometra and Lasiodiscus. South America Rainforest Brazil. The association between Cynometra and Colubrina in South America rainforests is not evident from a limited review. Cunha and Ferreira (2012) in a detailed floristic account of vegetation transects along the Xingu River in Pará Brazil mentioned three species of Cynometra (C. bauhiniifolia, C. cuneata, C. marginata, <1%) occurring in a forest with common Myrtaceae Myrciaria floribunda (23%), Psidium paraense (15%), Chrysobalanaceae Couepia cataractae (22%), and Verbenaceae Vitex duckei (10%), but no Rhamnaceae. Other species present in genera, which occur in African Cynometra forests, were Salacia (Celastraceae, Ghana, Kenya), or which occur with Lasiodiscus, were Terminalia (Cameroon), Pterocarpus (Cameroun), and undoubtedly others. In northeastern Brazil on the dry hills north of Óbidos (north of Manuas), Cynometra longifolia is reported with the cycad Zamia lecointei and other legumes such as Tachigalia grandiflora, Swartzia duckei, Ormosia cuneata, Peltogyne paradoxa, and species in Neotropical genera of other families, Cusparia trombetensis, Vochysia mapuerae, Bonnetia dinizii, Lacunaria sampaioi, Lophostoma dinizii, Ctenardisia speciosa, Mostuea brasiliensis, Macairea viscosa, Buchenavia corrugata, Ferdinandusa cordata, Pouteria speciosa, and Lepidocordia punctata (WWF, http://www.worldwildlife.org/ecoregions/nt0173). Venezuela and Mexico: Thorn Forests In thorn forests of northern Venezuela and Sinaloa Mexico are two related species of Colubrina that remarkably share many genera. Rubén Hernández Gil with reference to Pittier (1939) mentioned Colubrina reclinata with Caesalpinia coriaria, Acacia glomerosa, Cercidium [Parkinsonia, Caesalpinioideae] spinosum, Pithecellobium sp., Amyris balsamifera (Rutaceae), A. simplicifolia, Esenbeckia alata (Rutaceae), Plumeria alba (Apocynaceae), Ziziphus melastomoides (Rhamnaceae), Aspidosperma vargasii (Apocynaceae), Asp. cuspa, Mimosa sp., Myrospermum frutescens (Faboideae), Platymiscium polystachium (Faboideae), Ficus sp. (Moraceae), Croton scaber (Euphorbiaceae), Opuntia caracasana (or O. elatior, Cactaceae), and O. boldinghi. Shreeve and Wiggins (1964) in describing the vegetation in the Foothill Region of the southeastern margin of the Sonoran Desert, Río Yaqui region, mentioned Colubrina viridis (as C. glabra) to occur with Acacia brandegeana, A. cymbispia, A. occidentalis, Pithecellobium confine, Pithecellobium sonorae, Cercidium microphyllum, C. peninsulare, Esenbeckia hartmannii [as E. flava], Ziziphus sonorensis, Caesalpinia pulcherrima, C. platyloba, Plumeria acutifolia, Opuntia thurberi, and Op. fulginosa. It is interesting that Esenbeckia, a genus of ~20 species, occurs with Cynometra in the rain forest of Costa Rica and with Colubrina in both Venezuelan and Mexican thorn forests. Forchhammeria, a genus of ~10 species, also occurs at one of the thorn forest sites in Sinaloa with Colubrina viridis. In the Sonoran Desert of central Baja California is Colubrina californica that has been reported to occur with Pithecellobium and Mimosoideae Acacia mcmurphyi, Zapoteca formosa, and Erythea brandegeei (Arecaceae), various cacti Stenocerens thruberi, S. gummosus, Myrtillocactus cochal, Mammillaria dioica, M. lewisii, Lophocereus Ferocactus, Opuntia alcahes, and various shrubs Bursera microphylla, Jatropha cinerea, or subshrubs, Croton magdalenae, Nicotiana trigonophylla, Ambrosia carduacea, Anisacanthus (Hodgson & Rebman 9525, ASU-SEINet), also Celtis reticulata and Cercidium microphyllum (Rebman & Delgadillo 4425 UCR-SEINet) and seen as a tree to 10 m with Pachycormus, Yucca valida, Cercidium, Condalia globosa, Opuntia cholla, Larrea, Acacia greggii, Krameria grayi, Hymenoclea pentaleptis (Spjut & Marin 13753, US). Colubrina californica is related to C. cubensis (Johnston 1971, Sect. Serrataria). The association of Colubrina with Cynometra, other Detarioids in rain forests, and other genera in drier vegetation types, at disjunct locations, notably Africa, India, Malaya, and Caribbean Region, seem more than just coincidental. These reoccurring associations would appear to be related to vicariance events that may be traced back to Cretaceous forest types. The evolution of Colubrina species in adapting to drier vegetation would appear to have occurred independently in the Old World and New World. Thus, the strong similarity between Colubrina humbertii (Madagascar) and C. californica is probably one of convergent evolution. Cynometra-like fossils (Cynometroxylon) based on wood anatomy have been found in deposits dated from early Eocene in Tanzania, mid Eocene in Ethiopia, Oligocene in India and Ethiopia, and Miocene in Egypt, India (Cantrill et al. 2013; El-Saadawi et al. 2014), and include leaves assigned to the genus Cynometra (C. chaka) from the late Oligocene in northwestern Ethiopia (Pan et al. 2010). It is interesting that the related genus Sindora shows a similar paleogeographic distribution but with more records in Asia than in Africa (Prive´-Gill et al. 1999). This indicates that in the Paleogene there was a more northern and continuous occurrence of the African equatorial rainforest with geographical differentiation of the Detarioid forests. Actinosynnemal Associations with Colubrina Anticancer benzenoid anamycins (maytansinoids), namely colubrinol, were discovered in Colubrina texensis (Wani et al. 1973); however, maytansinoids had been known from actinomycetes before they were first isolated from vascular plants, species in the Maytenus ovata (Gymnosporia) complex (Celastraceae). They have since been found in Putterlickia verrucosa (Celastraceae), and Trewia nudiflora (Euphorbiaceae), all of which belong to the Rosid Clade (Cassady et al. 2004). Maytansinoids have also been found in mosses of the Thuidiaceae (Anomodon, Claopodium) and other moss families (Suwanborirux et al. 1990). A crude extract from a twig-leaf sample of Colubrina californica showed a greater increase in life span activity than C. texensis; Test/Control (x 100%) in a mouse leukemia assay (P388) for Colubrina texensis was 213% based on fractionation and isolation of the pure compound from twig-leaf material, in contrast to an initial fraction of a crude extract of C. californica that yielded a T/C of 232% (Wall, RTI unpubl. rpt.). The two species, which occur in North American deserts, are closely related. Maytansine, first isolated from Maytenus (Gymnosporia) serrata in Ethiopia, failed 2nd phase clinical trials during the late 1970's; consequently, pharmacological interest in Colubrina chemistry declined, although interest in the ansimitocins for treating cancer has continued (Spjut 2010). The maytansinoid compounds discovered in vascular plants, also known as ansamitocins primarily from actinomycetes in the genus Actinosynnema, led John Douros and Robert Perdue in 1977 or 1978 to obtain samples of the plants and their soil (Gymnosporia serrata, G. buchananii, Putterlickia verrucosa, Colubrina texensis) in the hope of isolating the microbial organisms responsible for the activity, because of the low yield of maytansine in the plant, but cultured microbes from samples apparently did not yield maytansinoids. Subsequently, strong circumstantial evidence for an actinosynnemal association was found to exist in the rhizophere of P. verrucosa (Wings et al. 2013). Additionally, it may be noted that actinorhizal plants—that are recognized by the development of endosymbiosis of the actinomycete Frankia with root nodules—are known for about 220 angiosperm species in the Rosid I clade (Santi et al. 2013). They are found in the Fagales (Betulaceae, Casuarinaceae and Myricaceae), Rosales (Rosaceae, Eleagnaceae and Rhamnaceae) and Cucurbitales (Datiscaceae and Coriariaceae) (Wall 2000; Pawlowski 2009; Franche and Bogusz 2011). Many clinical antitumor compounds isolated from plants are known to host microbial organisms that produce the same or related compounds. The microbes include cyanobacteria, eubacteria (especially actinobacteria), and fungi (Spjut et al. 1988; Cragg & Newman 2009; Spjut 2010, online report).
|
|
|
Colubrina californica |
Colubrina californica
|
|
Colubrina californica |
Colubrina californica |
|
Colubrina californica |
Colubrina californica Troop Scoutmasters and their Scouts, the Boy Scouts of America, participating in the collection of a large sample for chemical isolation and preclinical testing of the antitumor agent, colubrinol. |
|
Colubrina texensis |
Colubrina texensis Sample collected by the Volunteers
|