|
 
20.
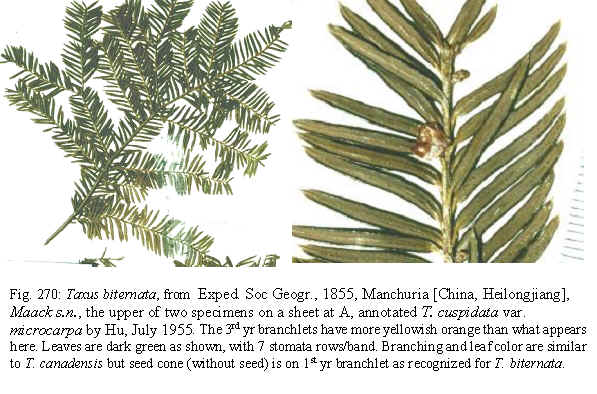
Taxus biternata
Spjut, J. Bot. Res. Inst. Texas 1(1): 264.
2007 (Figs. 2C, 145–146, 265–270).
Type: South Korea, Kyog[sang] Prov.: Kyongsan, Nemon-rei, common
or abundant, 15 Sep 1917, Wilson 10688, holotype: A! (Fig. 266,
with arillocarpia, abaxial leaf with marginal zone of 8–9 smooth
cells, 9 papillose cells, stomata 9 rows/band, and midrib lacking
papillae (isotypes K!, marginal leaf zone with 6 rows of bare cells
and 8 rows of papillose cells, 7 stomata rows, midrib with marginal
papillae; US!).
Taxus
microcarpa
(Trautv.) Spjut ined. (in adnot.: A, BH, BM, GH, K, NA, P)
Delicate-branch yew. Distribution: In
forests, 800–1400 m, China (NE, Manchuria), Russia (SE Region, North
Korea, South Korea, Japan. In
NE China dominant in “mixed broad-leaved deciduous and needle-leaved
evergreen forests” (Hou 1983). On N Hokkaido (Japan), “fairly common”
within a mixed conifer hardwood forest of Picea jezoensis,
P. glehnii, Abies sachalinensis, Populus maximowiczii, Acanthpanax
ricinifolius, Ulmus japonica, and Acer pictum (Wilson 1916,
as T. cuspidata). Common
in cultivation, including Cv. ‘Capitata’ and shrub forms misapplied
to T. media Rehder. At the Secrest Arboretum, apparently
spreading by seed among native deciduous hardwoods.
Trees or shrubs with erect trunks and horizontal
branches, to 30 m high; branchlets often short and much-divided,
subpinnately arranged but unequally divided, appearing ternately
divided—or with short delicate tertiary branchlets, horizontal or
weeping, yellowish green when young, yellowish orange with age;
bud-scales closely overlapping in 3–4 ranks, mostly persistent to the
3rd yr, thick, deltoid, concave, medially recurved and
incurved towards apex to form a cusp, with an obscurely thickened
mid-nerve, ca. 1 mm long, spreading from base of branchlets. Leaves
persistent on older twigs, or lacking, green upon drying, two-ranked to
apex, linear, straight to slightly falcate, 1–2 cm long, 1–2 mm
wide, 150–250 µm thick, pale green and convex above to a rounded
midrib that forms a channel along the base of the midrib, pale
yellowish-green and concave below to a rounded midrib, revolute near
margins 30–90ş in dried leaves, more notably revolute at upper
one-third of leaf; upper (adaxial) epidermal cells in T-sect.
elliptical, 10–15 µm tall, 25–40 µm wide; lower epidermal cells
similar or larger, 10–15 µm tall, 15–25 µm wide, numbering 11–18
between margin and stomata band, mostly rectangular, or incrassate near
the stomata band, 3–7× l/w except quadrate in 1–3 rows near
margins, epapillose entirely across the marginal region, or marginal
region often partially papillose, often epapillose on (6-) 8–18 cells
in from the margins, occasionally with obscure papillae on midrib,
papillae usually more prominent on marginal cells bordering stomata
band, in 2 opposite rows; stomata bands broader than the marginal
region, with 7–13 (-16) stomata
rows/band. Male bud cones
globose, ca. 1 mm diam.; scales 4-seriate; sporophylls ca. 14, united
into a terete, smooth or obscurely ribbed column, thickened at apex,
spreading shortly above, each branch bearing 8–10 lobed, cucculate
sporangia. Female cone scales 4–5 seriate; aril red or pink with tinge
of white, with a deep cup, drying dark purple; seed subglobose,
obscurely angled where tapering to apex in upper half, 4 mm long, 2–4
mm diam.
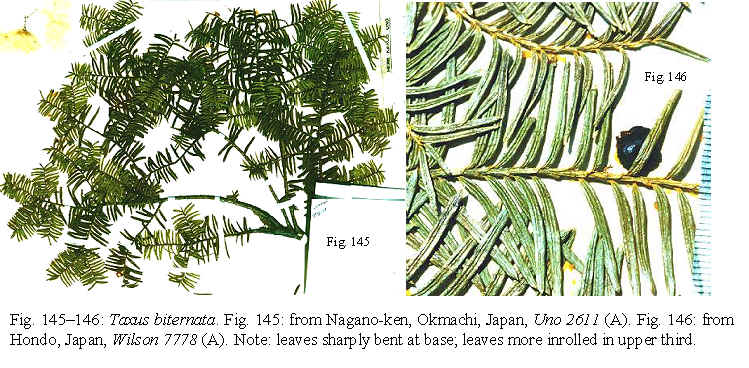
Taxus biternata is
easily identified by its tree habit with an erect trunk and horizontal
diffuse branching (Fig. 33), and by the much divided slender branchlets
with two ranked leaves that spread horizontally (Fig. 31, 32). The
horizontal diffuse branching not only distinguishes this from T. cuspidata, which
has long ascending or recurved branches, but also from a shrub
yew originally described as T. baccata var. microcarpa
Trautvetter.
A detailed study by Kolesnikov (1935) showed that T. biternata—regarded
by him as T. cuspidata—and the shrub yew are parapatric in which
they are distinguishable by morphological and ecological characteristics.
The shrub
yew, T. umbraculifera var. microcarpa, is similar to T.
canadensis in layering, but differs in its flat-topped radial
growth—as illustrated by Kolesnikov (1935), and by the much smaller
paler seed, shaped like a “Hershey Kiss.”
Additionally, I include other variation in this taxon based on
phyllotaxy.
Occasional
specimens of T. canadensis from North America (e.g., Travis
119, Maine, PH), Estonia (e.g., Lundström 742, S), and
others from Europe (e.g., Handel
Mazzetti, Mt. Olympus, Greece, K) and SW Asia (e.g., Davis 13667,
Turkey, K), treated as T. baccata var. washingtonii (Hort.
ex Bot. Berjianus, annot. Florin, S), are similar to T. biternata
in features such as the two ranked leaf arrangement, the linear leaf shape, and leaves
appearing more strongly revolute in the upper third. These differ, nevertheless, by color of branchlets—appearing dark
green to yellowish green in T. canadensis and yellowish orange in T.
biternata. Taxus
canadensis also differs from T. biternata in a number of
features such as seed developing
on the 2nd
yr branchlets, isodichotomous branching,
leaves overlapping more closely along one side of branchlet in pressed
specimens, falcate leaf shape, and more papillose cells across marginal
zone of leaves. Despite these differences, the similarity in branching
and phyllotaxy is still
remarkable.<
In
SE Manchuria T. biternata appears to hybridize with T.
umbraculifera var. microcarpa and var. umbraculifera.
Plants with linear leaves (10× l/w or more) that are strongly recurved in upper
third are referred to T. biternata. Those with relatively short
leaves (oblong, 5–8× l/w) are referred to var. microcarpa. Taxus biternata
can be difficult to distinguish from Taxus cuspidata, the
name that many authorities might apply to this species.
However, the recurved branchlets and upwardly pointed leaves of T. cuspidata
are worthy of separate taxonomic status as emphasized by others (Bailey
1933; Hatfield 1921; Rehder 1940). The evolutionary significance of
secund leaves is discussed under T. caespitosa.
Branches of T.
cuspidata are thicker and more rigid, compared to those of T. biternata,
and the dried leaves in specimens from Japan and Korea are more
uniformly recurved to revolute along margins, whereas those of T. biternata
are more revolute in the upper third (appearing as if pinched slightly)—a
useful taxonomic feature for identifying this species.
The lectotype of T. cuspidata,
and other specimens from Japan, also have conspicuous
carinate bud-scales that Siebold and Zuccarini (1870) considered diagnostic for
this species, in contrast to smaller scales of T. baccata, but
this does not hold true as a clear diagnostic feature for separating all yews in E
Asia from those in the Euro-Mediterranean as these authors and Rehder
(1940) have indicated (see also Cope 1998 for illustrations of bud-scale
features); the differences between the type specimens of T. baccata and T.
cuspidata seem less significant when many specimens are taken into
consideration across the geographical range of the species, while cuspidate axillary bud-scales
are also seen in specimens from Europe. Taxus
biternata
is common in cultivation as a tree or shrub. I have
observed both male and female trees growing apparently wild at the
Secrest Arboretum where their origin and identification have been a
mystery (Kenneth Cochran, pers. comm. Nov. 1992, accompanied by J.
Thieret, M. Hils). It is also cultivated in other arboreta such as
in Sweden (Anderberg s.n., S), and at the Forestry Research
Institute in Seoul (Republic of Korea). The tree forms are easily
recognized, whereas shrub forms seem to intergrade with
T. cuspidata. This species has also been referred to as T. media
based on specimens I have received from Phyton (Ithaca, MY), and those
labeled at the Secrest Arboretum (e.g., cv 'T. media Green
Wave', A29-80). While leaves of T. biternata
are distinctly “two-ranked,” a character feature emphasized by
Rehder (1923) for recognizing T. media, the leaves in the type of T. media
differ in having darker and thicker (lip-like appearance) margins, which
are not recurved (compare close-up photos in key), and they also
frequently crisscross instead of spread mostly parallel to each
other. Specimens from the Forestry Research Institute in Korea show
additional variation in bark that merits further study. The cv. 'Capitata'
as shown in Hatfield is probably this species as I have also determined
from a specimen at the Secrest Arboretum that had this name.
Representative Specimens—Russian Federation—Far East Region:
Pryanyk For. Div., Zalese Village, silver fir-cedar-broad-leaved forest,
B. Cerereu (in Russian, A). ). [Azerbaijan?] Kura Mts.? in
Russian #75 (P). Manchuria: Northern, Sochintzest,
forest, small trees 20 Sep 1931, Skvortzov s.n. (A). Sikhote-Alin:
R.a., foothills of Mount Hezalaza, valley of River Beryozovoy, mixed
forest, Lyubarsky 2 (in Russian, A). Manchuria (Mandshuria) SE:
Ex herb. hort. bot. Petro. yr 1860, Maximowicz (A). China—Jilin
(Kirin), 5 Sep 1931, C. H. Chen 539 (A); Northern China {Shaanxi:
Tai-pei-shan fide Rehder & Wilson in Sargent 1914],
Purdom s.n. (GH); Mandshuria SE, ex herb. hort. bot. Petropolitani,
1860, Maximowicz, iter secundum (A, US). [South] Korea.
Kyog[sang] Prov.: Kyongsan, Nemon-rei, common or abundant, Wilson
10519 (A, BM, US), Wilson 10688 (A, BM); N. Heian Prov.,
O.G.M. Co. Mines, Pukchin & Takkari, 833–1000 m, not uncommon in
moist forests, 22 Jun 1917, Wilson 8685 (A, K, US); Shinkabachin
Heizanchien to Ehoshin, Kankyo-N Heian divide, rare, 5 Sep 1917, Wilson
9097 (A); Seoul, East Palace Park 24 Sep 1905, Jack s.n. (A).
Japan—Hokkaido: Cosl Mines, Utishini, tree 40–5 x 2
(degree sign, ft?), 20 Sep 1892, Sargent s.n. (A); Aza-akaigawa
in Morin-machi, 42.0N 140ş39'E, near stream in open woodland, 200 m,
tree, 20 ft, seeds embedded in reddish aril, Meyer et al. 19261
(NA); Teshikaga-Machi, 3.2 km SE of Lake Kusharo, road 243,
Kawakami-gun, Kushiro, 43.35 N, 144.23 E, Meyer et al. 19112
(NA); Hokkaido, Kitami prov., common in moist woods, tree 15 m x 1.5 m,
17 Aug 1914, Wilson 7399 (A).
Honshu: Sernja prov., Yamanaka on Fuji-san, abundant, tree
6-13 m x 1.5-2.6 m, 4 Nov 1914, Wilson 7778 (A, K); Kai prov.,
around village of Nakaihinsen, common hedge 18 Sep 1914, Wilson 7544
(A); Nagano-ken, Okmachi, 27 Aug 1951, Uno 2611 (BH, A);
Yokohama, ex Herb. Hort Petro., Maximowicz (P); Kamikawa, Nitzelius
(S: C-2111); Tokyo Pref.: Oizuni, Nepymawku Makino 43775 (S:
C-2111); Mt. Kiyosumi, Makino 43779 (S: C- 2122); Sapporo, Yezo,
21 Jun 1903, Arimoto s.n. (A). Hida, Takayama (A); Shimano
Togakushi, 16 Sep 1907 (US:1311890). Cultivation—Ohio,
Secrest Arboretum: tree growing naturally outside the arboretum yew
plot among native hardwoods and introduced conifers, 10-15 m high, bole
20–5 cm diam., Spjut 12179 (wba).
Japan: cult., Mino Prov., Shiota 4441 (A); Yokahama,
ex Herb. hort. bot., Petro., Maximowicz, 1862 (A); Kagoshima
Pref., Mt. Takahuma, in forest, tree to 5 m, Hatusima 13858 (A).
|